Content Benchmark P.8.C.5 conduction, convection, and radiation. E/S
advertisement

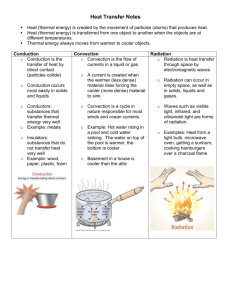
Content Benchmark P.8.C.5 Students know heat energy flows from warmer materials or regions to cooler ones through conduction, convection, and radiation. E/S Heat Heat is the transfer of thermal energy from an object of higher temperature to one of lower temperature. Transfer of heat is related to the motion of molecules within objects. The Kinetic Theory of Matter states that all forms of matter (solids, liquids, or gases) are composed of atoms or molecules that are in constant motion. In part, because of this constant motion, all objects have internal energy. Whenever heat is transferred to a substance, the atoms move faster and faster. When heat is transferred from a substance (more commonly called cooling), the atoms move slower and slower. The "average motion" of the atoms that we sense is what we call temperature. When a temperature difference occurs in solid objects, the faster moving particles at the higher temperature end collide with a slower moving particle at the lower temperature end. Some energy is transferred to the slower moving particle, increasing the speed of that particle. With millions and billions of particles colliding into one another heat is also transferred from the high temperature region to lower temperature region. Eventually, the temperature will be the same throughout the object and heat will no longer be transferred. To learn about internal energy and heat, go to http://www.unf.edu/~jgarner/specificheat.html. For further information on internal molecular motions and the Kinetic Theory of Matter, refer to TIPS Benchmark P.8.A.1. For further information on internal molecular motions and the states of matter, refer to TIPS Benchmark P.8.A.5. Temperature Temperature is the measure of the average kinetic energy of the particles in a sample of matter. The faster the particles move the more kinetic energy the object has and the higher the temperature. On the other hand, if the particles in an object move more slowly, their average kinetic energy will decrease, and their temperature will also decrease. Temperature is used to describe how hot or cold an object is. The temperature of an object depends on how fast its atoms and molecules oscillate. At absolute zero, these oscillations are the slowest they can possibly be. Even at absolute zero, the motion does not completely stop. Temperature and heat are not the same. Temperature is the average motion of atoms and molecules. Heat is the energy that transfers due to these temperature differences. Heat is always transferred from warmer to cooler substances. To learn more about temperature, go to http://eo.ucar.edu/skymath/tmp2.html Three Modes of Heat Transfer There are three modes of heat transfer: conduction, convection, and radiation. Any heat exchange between objects occurs through one of these modes or a combination of them. Conduction is the transfer of heat through solids or stationery fluids. Convection uses the movement of fluids to transfer heat. Radiation does not require a medium for transferring heat; this mode uses the electromagnetic radiation emitted by an object for exchanging heat. To learn more about the modes of heat transfer, go to http://www.physchem.co.za/Heat/Transfer.htm Conduction Conduction is the transfer of heat through direct particle to particle contact when a temperature difference exists within a certain material. Conduction can also occur from one material to another when the two materials are in direct contact and at different temperatures. Because of the relatively close spacing between atoms and molecules in a solid, conduction is a dominant mode of heat transfer in this state of matter. If the temperature of one side of a solid material is higher than the other, the atoms and molecules on the higher temperature end will have more energy than on the cooler end. Through the more vigorous particle motions associated with this higher temperature side, energy is transferred to neighboring atoms and molecules through collisions, thereby causing an energy increase in these adjacent particles. Energy is transferred along the solid material through these collisions and the temperature of the object will increase until all molecules have essentially the same energy. At this point, heat transfer ceases because there is no longer a difference in temperature and we call this state thermal equilibrium. To learn more about conduction, go to http://physics.nmt.edu/~raymond/classes/ph13xbook/node225.html The image below demonstrates that if the temperature is greater in one part of a solid object, the electrons within the atom are more excited (i.e., they have more kinetic energy). This causes molecules to collide and increases the energy of slower moving molecules. In solids, atoms are bound to each other by a series of bonds. When there is a temperature difference in the solid, the hot side of the solid experiences more vigorous atomic movements. The vibrations are transmitted to the cooler side of the solid. Eventually, they reach equilibrium, where all the atoms are vibrating with the same energy. Figure 1. The red particles represent higher temperature molecules and the grey areas indicate cooler temperature molecules. (From http://www.qrg.northwestern.edu/projects/vss/docs/thermal/1-how-does-heat-move.html) Properties of conductors and insulators Some substances conduct heat more easily than others. In general, solids are better conductors than non-moving liquids, and liquids are better conductors than gases. Metals are very good conductors of heat, while still air is a poor conductor of heat. The ability to transfer heat within an object is called thermal conductivity. Thermal conductivity varies for different materials. Gold, silver, and copper have high thermal conductivity. These materials are also good conductors of electricity. If an object is not a good conductor of heat or electricity it is an insulator. An insulator is made of materials that have low thermal conductivity like wood, plastic, or even air which is a gas. A good insulator does not allow heat to pass through it easily. Your winter jacket is designed to trap the heat from your body and keep it from escaping into the open air. Homes and buildings are insulated to keep the warm air inside and the cold air outside. Insulation also reduces the amount of heat that moves into a cooled building from the outside, keeping the inside of the building cooler. To learn more about thermal conductivity, insulators, and conductors, go to http://phun.physics.virginia.edu/topics/thermal.html Convection Convection uses the motion of fluids to transfer heat. In a typical convective heat transfer, a hot surface heats the surrounding fluid, which is then carried away by fluid movement. The warm fluid is replaced by cooler fluid, which can draw more heat away from the surface. Since the heated fluid is constantly replaced by cooler fluid, the rate of heat transfer is enhanced. This cycle results in a continuous circulation pattern and heat is transferred to cooler areas. The warmer portions of the fluid are less dense and therefore, they rise. At the same time, the cooler portions of the fluid fall because they are denser. In liquids and gases, convection is usually the most efficient way to transfer heat. Figure 2. Schematic diagram of the convection mode of heat transfer. (From http://www.antonineeducation.co.uk/Physics_GCSE/Unit_1/Topic_1/topic_1_how_is_heat_transferred.htm) Forced convection uses external means of producing fluid movement. Forced convection is what makes a windy, winter day feel much colder than a calm day with the same temperature. The heat loss from your body is increased due to the constant replenishment of cold air by the wind. Natural wind and fans are the two most common sources of forced convection. For further information on convection, go to http://www.qrg.northwestern.edu/projects/vss/docs/thermal/1-how-does-heat-move.html Radiation Radiation is defined as the transfer of energy in the form of electromagnetic waves. These waves do not require a medium (solid, liquid, or gas) for energy propagation, so electromagnetic radiation can transfer heat through space. Sunlight is a form of radiation that has traveled 93 million miles of empty space to reach Earth. Both conduction and convection require matter to transfer heat. Radiation does not. The use of a microwave to transfer heat a cup of tea is one example of radiation. The water temperature increased due to transfer of heat by microwaves, a form of electromagnetic radiation. Radiation from the Sun is the primary energy source for Earth’s atmosphere. Secondarily, convection currents move greater temperature air around the Earth, and this difference between warm and cold air provides the energy needed to create weather. For further information on radiation and it role in the Earth’s energy budget, go to http://eesc.columbia.edu/courses/ees/climate/lectures/radiation/index.html Summary Conduction, convection, and radiation all involve the transfer of heat energy. The differences between these processes are that each is associated with a particular type of medium and mode of heat transportation. Radiation involves the transport of heat through electromagnetic waves. Convection involves the transport of heat through fluids (liquids or gases). Conduction involves the transport of heat in solids or stationary fluids. We have looked at three types of heat transfer - conduction, convection, and radiation separately. However, it is important to note that often heat transfer occurs in two or even all three of the processes simultaneously. A stove and oven are perfect examples of the different kinds of heat transfer. If you boil water in a pot on the stove, heat is conducted from the hot burner through the base of the pot to the water. Heat can also be conducted along the handle of the pot, which is why you need to be careful picking the pot up, and why most pots don't have metal handles. In the water in the pot, convection currents are set up, helping to heat the water uniformly. If you cook something in the oven, on the other hand, heat is transferred from the glowing elements in the oven to the food via radiation. For further information on how heat energy moves, go to S-Cool! – GCSE Physics, an animated and interactive website found at http://www.scool.co.uk/topic_quicklearn.asp?loc=ql&topic_id=5&quicklearn_id=2&subject_id= 16&ebt=5&ebn=&ebs=&ebl=&elc Content Benchmark P.8.C.5 Students know heat energy flows from warmer materials or regions to cooler ones through conduction, convection, and radiation. E/S Common misconceptions associated with this benchmark 1. Students incorrectly believe that heat and temperature are the same thing. Temperature is the measure of the average kinetic energy of the particles in a sample of matter. Heat is the transfer of energy due to a difference in temperature. As heat energy is transferred, temperatures may increase or decrease within objects. If heat is transferred to an object, the temperature of that object will increase. If heat is transferred from an object to another, the temperature will become lower for the object that is transferring heat (provided that no energy is being transferred to the higher temperature object). Higher temperatures mean that the molecules are moving (vibrating and rotating) with greater kinetic energy. If we take two objects which have the same temperature and bring them into contact, there will be no overall transfer of energy between them because the average kinetic energies of the particles in each object are the same. But if the temperature of one object is higher than that of the other object, there will be a transfer of energy from the hotter to the colder object until both objects reach the same temperature. Temperature is not energy, but a relative measure of “hotness” and “coldness” in an object. Heat is a mechanism by which energy is transferred from one object to another. For animations and simple explanations to help differentiate between heat and temperature go to, http://id.mind.net/~zona/mstm/physics/mechanics/energy/heatAndTemperature/heatAndTempera ture.html 2. Students incorrectly think that heat is a fluid or that heat is energy. Heat is not a property of matter, either as some kind of fluid substance that flow from one object to another or as energy that is inherent within an object. There are really only two kinds of energy: kinetic energy and potential energy. Particles within an object have both kinetic energy, because they are in constant motion, and potential energy, predominantly due to particles position within the electromagnetic fields in the object (i.e., the binding energy that holds the particles together). The sum of these kinetic and potential energies is sometimes called the internal energy within an object. Because of the casual language used in textbooks and science websites, students will confuse this internal energy with heat. But, heat is not internal energy; rather heat is the energy that is transferred between objects due to a difference in temperature. Heat is transferred through conduction, convection, and radiation, where a greater internal energy in a warmer region is transferred to a lower temperature region. This transfer increases the internal energy (again, the kinetic and potential energy of the particles in the object) of the lower temperature object. This often results in an increase in temperature or change of state in the lower temperature object. To learn more about internal energy and it relationship to heat transfer, go to http://www.science.uwaterloo.ca/~cchieh/cact/c120/conserve.html. 3. Students incorrectly believe that heat is transferred from colder regions to warmer regions. How often have you heard someone mistakenly say, “Close the refrigerator door, you are letting all of the cold air out?” The truth of the matter is that heat is transferred from a high temperature region to a low temperature region. This transfer of heat will continue until there is no temperature difference, which is also known as equilibrium. To learn more about this misconception, go to http://www.ftexploring.com/energy/heatflow.htm 4. Students incorrectly believe that heat rises. Because heat is not matter, it is not correct to say it rises or falls. Heat is the transfer of energy due to differences in temperature. As the air temperature increases due to transfer of heat, the molecules that make up the gas (air) begin to move faster and faster, and therefore, move further apart. This lowers the density of the air. The more dense cooler air forces the less dense hot air up. One example is a hot air balloon: the heated air becomes less dense and because it is confined in the balloon, the average density of the balloon eventually becomes less than air surrounding it. More information about the “rising heat” misconception can be found at http://coolcosmos.ipac.caltech.edu/cosmic_classroom/light_lessons/thermal/transfer.html 5. Most students are familiar with the term Absolute Zero but incorrectly believe that this temperature can be reached in space. Absolute zero is the point where no more heat can be transferred from a system and particle motion completely stops. You cannot reach absolute zero because it impossible to completely stop molecular motion. In laboratory experiments, scientists have uses lasers to almost stop particle motion, resulting in temperatures close to absolute zero. At these very low temperatures, another state of matter exists, called Bose-Einstein condensate. To learn more about this misconception and Bose-Einstein condensate, go to http://www.colorado.edu/physics/2000/bec/temperature.html Content Benchmark P.8.C.5 Students know heat energy flows from warmer materials or regions to cooler ones through conduction, convection, and radiation. E/S Sample Test Questions 1. If you place three ice cubes in a glass of water at room temperature, which of the following will occur? a. Heat transfer will occur from the higher temperature liquid to the lower temperature ice, which in turn, will melt the ice b. Heat transfer will occur from both the ice to the liquid and liquid to the ice, resulting in colder liquid water and melted ice. c. Heat transfer will occur from the colder temperature ice to the higher temperature liquid, which in turn, will make the liquid water colder. d. Heat will not transfer to the liquid or ice because there are no solid surfaces in contact. 2. When heat is transferred to an object, the particles in that object a. may move more slowly b. may stop moving completely c. may move more quickly d. may either move more quickly or more slowly 3. A measure of the average kinetic energy of the individual particles in an object is called a. heat b. thermal energy c. internal energy d. temperature 4. The transfer of energy from a warmer object to a cooler object is called a. heat b. thermal energy c. internal energy d. temperature 5. Heat transfer from one particle of matter to another through direct contact is called a. conduction b. convection c. radiation d. insulation 6. Hot air from vents will increase the temperature in a room through the heat transfer process called a. insulation b. radiation c. conduction d. convection 7. Heat is the transfer of energy a. from lower temperature objects to higher temperature objects b. both from cold objects to hot objects and hot objects to cold objects c. from higher temperature objects to lower temperature objects d. equivalent to the temperature of an object 8. When you use a microwave oven to bring water to a boil, energy is transferred from the oven to the water in a process called a. conduction b. convection c. radiation d. induction 9. Heat is transferred from the Sun to the Earth through a. thermal emission b. radiation c. convection d. conduction 10. The air near the ceiling of a room is at higher temperature than the air near the floor because a. heat rises, and as it rises, will cool the air near the floor b. the hotter air is less dense, rises, and is replaced by cooler air c. the hotter air radiates its heat upward and cools the air near the floor d. the colder floor transfers its heat to the air and it expands upward Content Benchmark P.8.C.5 Students know heat energy flows from warmer materials or regions to cooler ones through conduction, convection, and radiation. E/S Answers to Sample Test Questions 1. (a) 2. (c) 3. (d) 4. (a) 5. (a) 6. (d) 7. (c) 8. (c) 9. (b) 10. (b) Content Benchmark P.8.C.5 Students know heat energy flows from warmer materials or regions to cooler ones through conduction, convection, and radiation. E/S Intervention Strategies and Resources The following is a list of intervention strategies and resources that will facilitate student understanding of this benchmark. 1. Convection Current This is a laboratory activity that can be used in the classroom to demonstrate convection currents in water. The demonstration explained on this website gives a simple and visually appealing way to demonstrate convection currents in water. The warmer water rising through cooler water creates turbulence effects that bend light, allowing a swirling shadow to be projected onto a screen. To access this demonstration, go to http://www.exploratorium.edu/snacks/convection_currents.html 2. Investigating Radiant Heat Energy This is a laboratory activity to use in the classroom to investigate the properties of radiant heat energy. Students will compare the temperature of water in a black can to the temperature of water in a white can during this laboratory activity. The students will create a time/ temperature graph to display their data. This investigation is found at http://educ.queensu.ca/~science/main/concept/is/i02/I02lams3.html 3. Popcorn Lesson: Three methods of heating popcorn This website explains that there are three ways to cook popcorn: an air popper, a microwaveable bag of popcorn, and a stove with a pan of oil on it. These can be used to demonstrate how popcorn can be made. As each method is demonstrated the students will decide whether they are observing conduction, convection, or radiation. To download this lesson go to http://outreach.physics.utah.edu/labs/atmosphere/popcorn.html 4. Quiz: Heat Game Quia is short for Quintessential Instructional Archive. This website provides a variety of teaching tools that can be used in the classroom. On this site you can create educational games and activities, quizzes, surveys, Web pages, and more. This link will take you to a free online quiz. Students answer each question and are provided with instant feedback before moving onto the next question. The quiz can be found at http://www.quia.com/pop/10875.html?AP_rand=1959887284 5. Heat Transfer Mini Quiz Ed’s Online Activities is a website that contains sample learning materials for various topics. This link will take you directly to an online quiz. There are 20 questions on the Heat Transfer Quiz and your students will have to complete each question before moving onto the next. You can use this activity to introduce the topic or to review it. To access the quiz, go to http://www.edukate.net/ed1_files/heat_transfer.swf 6. Cooking Cookies with Solar Energy This website is from the Teachers Domain and includes a video segment adapted from ZOOM. The cast tests two homemade solar cookers to determine which one can cook a "s'more" faster. Both designs exploit the fact that heat is transferred in three ways: by conduction, convection, and radiation. Though one cooker performs better than the other, they both outperform the experiment's control setup. There are discussion questions and background information to use with this activity. To access this activity go to, http://www.teachersdomain.org/resources/phy03/sci/phys/mfe/zsolar/index.html. 7. NOVA: Absolute Zero This is a link to a NOVA movie titled: Absolute Zero. This movie tells a story of the harnessing of cold and the race to reach the lowest temperature possible. The website contains the two hour movie to view online and provides the user with a transcript of the video. At PBS.org students can enter a virtual lab and see how close they can get to absolute zero, make their own temperature scale and more. Information about the movie, including an entire program transcript, is found at http://www.pbs.org/wgbh/nova/transcripts/3501_zero.html To access the virtual laboratory, go to http://www.pbs.org/wgbh/nova/zero/scale.html