16.2B Change in Enthalpy for a Reaction (4) Lab Report = 131
advertisement

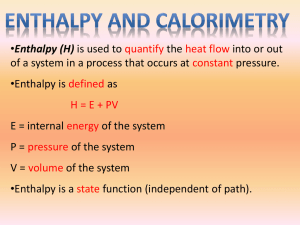
Laboratory 16.2B Change in Enthalpy for a Reaction (4) Lab Report = 131 Introduction: (5) When a chemical reaction takes place, chemical bonds in the reactants are broken and new chemical bonds in the products are formed. Energy is always absorbed in the breaking of bonds and always released when bonds are formed. If the energy required to break old bonds is less than the energy released in forming new bonds, the difference in energy is given off, and the reaction is said to be exothermic. The enthalpy change for an exothermic reaction is given a negative sign to indicate that energy flows from the system. Changes in enthalpy for reactions can be calculated by using tables of standard enthalpies of formation of compounds. The standard enthalpy of formation of a compound, ∆Hƒ˙, is the energy change involved in the formation of one mole of the compound from the elements when all reactants and the product are in their standard states. Standard conditions are 298˙K and a pressure of one atmosphere. 1 1 H2(g) + Cl2(g) HCl(aq) ∆Hƒ˙= -84.0 kJ/mol 2 2 The equations can be rearranged, which is called Hess’s Law, to obtain the final equation. Mg(s) + 2HCl(aq) H2(g) + MgCl2(aq) The standard enthalpy of formation (∆Hƒ) for the final equation can be found by using the enthalpies from the two equations. In this experiment, precautions must be taken to retain the heat energy in such a sway that the immediate environment retains the energy so that an accurate accounting can be made. A calorimeter is used to isolate the reaction from the surroundings. In this experiment, you will measure the amount of energy released by the production of hydrogen gas, H2, from the reaction between magnesium metal and hydrochloric acid and compare the experimentally determined result with the value calculated using the following standard enthalpies of formation data. You will also analyze the experiment to account for any differences. The heat released in the above reaction is a direct measure of the heat of formation, ∆Hƒ˙, of the magnesium ion, Mg2+. This is because the heats of formation of all the other species are zero. By definition, the heat of formation for any element in its most stable state at standard conditions is defined as being zero. The amount of energy released in the reaction can be calculated from the mass of the water rises. The amount of energy released in the reaction can be calculated from the mass of the water in the solution, the change in temperature, and the specific heat of water (4.18 J/g-ºC). Mg(s) + Cl2(g) MgCl2(s) ∆Hƒ˙= -640.7 kJ/mol Energy released = (mass) x (specific heat) x (change in temperature). ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• Objectives: (2) 1. Calculate the theoretical change in enthalpy for the formation of hydrogen gas. 3. Compare the experimental and theoretical values. 2. Determine the enthalpy change experimentally. ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• Materials: (5) Polystyrene foam cup calorimeter with lid Temperature probe system 50-mL graduated cylinder laboratory apron safety goggles •••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• Reagents: Magnesium ribbion 2.0 M HCl Laboratory 16-2 Page 1 ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• Procedure: 1. Put on your laboratory apron and safety goggles. 7. Add the HCl to the calorimeter and record the mass to the nearest ten-thousandths of a gram. 2. Open up Logger Pro to get the thermometer readings, same as Expt 15.2 8. Add the magnesium ribbon to the calorimeter and quickly attach the lid. Make sure the temperature probe is through the lid. Gentle swirl the calorimeter to distribute the heat 3. Assemble the calorimeter. Take two Styrofoam cup’s and one plastic lid. Insert one cup inside to the other to insulate. Carefully push the temperature probe through the calorimeter lid and position the tip so that it is about 2 cm from the bottom of the cup 9. Click “Collect” on logger pro. Watch the temperature constantly, because you want the highest temperature reading. Record the highest reading. 3. Mass the entire calorimeter (lid and cups) to the nearest ten-thousandths of a gram. 10. Dispose of the materials into the acid bottle by the sink. 4. Take a 10-15 cm length of magnesium ribbon and clean it with a piece of steel wool if needed. 11. Thoroughly rinse out the calorimeter. Dry it thoroughly as well. 5. Mass the cleaned piece of magnesium ribbon to the nearest ten-thousandths of a gram and record 12. Repeat the procedure two more times. 9. Before leaving the lab, wash your hands thoroughly. 6. Measure 45-50-mL of 2.0 M hydrochloric acid and record the temperature of the HCl. ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• (40)Data and Observations: Trial 1 Trial 2 Trial 3 Mass of Mg (g) Mass of Calorimeter & HCl soln. (g) Mass of empty calorimeter (g) Mass of HCl soln. (g) Final Temp (°C) Initial Temp (°C) Change in Temp (°C) Moles of Mg Total Joules lost by reactants Total joules gained by calorimeter kJ per mole Mg (expt) kJ per mole Mg (theoretical) Percent error Avg Percent Error (20) Stamp………………… Laboratory 16-2 Page 2 ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• (20) Calculations: (4) 1. Calculate the number of moles of magnesium. (4) 2. How much energy was released into the HCl solution from production of hydrogen gas? Assume the density and specific heat of HCl is similar to water. (2) 3. How much energy was gained by the calorimeter? What assumption must be made for this value to be correct? (4) 4. Determine the experimental value for the change in enthalpy per mole of magnesium. (How much heat is produced per mole?) (4) 5. Calculate what percent error between the theoretical (prelab) enthalpy and your experimental value. (2) 6. Calculate the average percent errors. ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• (10) Analysis: (2) 1. Why is the temperature of the 2.0 M hydrochloric acid measured in the graduated cylinder instead of the cup? (4) 3. Analyze your results and suggest two reasons for the difference between your results and the theoretical answer. (4) 2. The negative chloride ion is not considered to be part of this reaction. What factors allow us to ignore the chloride ion? . ••••••••••••••••••••••••••••••••••••••••••••••••••• (5) Conclusion: Analyze your data and explain if the data supports Hess’s Law. If not, explain what factors caused the difference. Laboratory 16-2 Page 3