Chemistry Study Guide: Matter and Energy - High School
advertisement

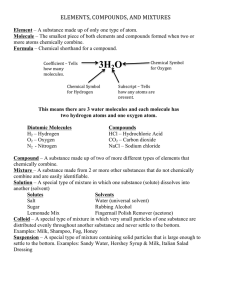
Name:______________________________________________________Pd:_______Date:_________________ Chapter 3: Matter and Energy – Study Guide *Do not forget about the extra resources available to help you study and prepare for this test! Textbook pages 81- 93 (answers to odd questions in the back of the book) Mastering Chemistry Study Area: http://www.pearsonmylabandmastering.com/northamerica/ Miss Marnik’s Website: http://www.northallegheny.org/Page/16665 Labs, problem packets, and homework assignments Section 1 – Classifying Matter: (text pages 57-62) Need to be able to: Define matter, atoms, molecules, element, compound, and mixture. Classify matter as and distinguish between solid, liquid, or gas. Classify matter as element, compound, or mixture. Practice Problems: 1. Match the following terms with the phrases below: a. State of matter in which atoms or molecules pack close to each other in fixed locations 1. heterogeneous mixture 2. homogeneous mixture b. State of matter in which atoms or molecules are packed close together but are free to move around and by each other 3. liquid 4. mixture c. Substance composed of only one type of atom or molecule 5. pure substance d. Matter composed of two or more different types of atoms or molecules combined in variable proportions 6. solid e. Mixture that has two or more regions with different compositions f. Mixture that has the same composition throughout 2. Which of the following can be classified as matter? a. atoms c. wind e. potential energy g. carbon b. gas d. liquids f. h. light temperature 3. Classify each type of matter as a pure substance or a mixture. If it is a pure substance, classify it as an element or a compound. If it is a mixture, classify it as homogeneous or heterogeneous. a. limestone (CaCo3) e. Chex mix b. orange juice with pulp f. Table salt c. helium g. Brass d. Pacific ocean h. oxygen 1 4. Distinguish between a crystalline solid and an amorphous solid. 5. Would the following pictures be classified as pure substances or as mixtures? 6. Describe the differences between solutions, suspensions, colloids, and alloys. Section 2 – Changes in Matter: (text pages 63-68) Need to be able to: Distinguish between physical and chemical properties. Distinguish between physical and chemical changes. Apply the Law of Conservation of Mass. Practice Problems 7. Match the following terms with the phrases below: a. Property that a substance displays without changing its composition 1. chemical change b. Property that a substance displays only through changing its composition 2. chemical property c. Change in matter with no change in composition d. Change in composition of matter e. Substance present in a chemical reaction before the chemical change f. Substance present in a chemical reaction after the chemical change g. Process by which one or more substances transform into different substances via a chemical change h. Separation technique that involves pouring off a liquid from a solid i. Separation technique that separates liquid mixtures by heating them to their boiling points. j. Separation process in which a liquid and solid mixture is poured through filter paper 3. chemical reaction 4. decanting 5. distillation 6. filtration 7. physical change 8. physical property 9. product 10. reactant 2 8. Decide if each of the following is a physical or chemical property: a. the explosiveness of hydrogen gas c. the shiny appearance of silver b. the bronze color of copper d. the ability of dry ice to sublime 9. Decide if each of the following is a physical or chemical change: a. Ice melting c. A candle burning b. Silver tarnishing to a dull finish d. Puddles evaporating in the sun 10. List four observations that would be evidence that a chemical reaction has taken place. 11. Would the following picture represent a physical or a chemical change? Explain your answer. 12. Explain how paper chromatography separates mixtures. 13. Potassium iodide reacts with chlorine to form potassium chloride and iodine. If 7.16 g of potassium iodide reacts with 1.53 g of chlorine to form 3.22 g of potassium chloride, how many grams of iodine form? 3 Section 3 – Energy: (text pages 68-72) Need to be able to: Recognize the different forms of energy. Identify and convert among energy units. Distinguish between exothermic and endothermic reactions. Practice Problems: 14. Match the following terms with the phrases below: a. Energy can be neither created nor destroyed 1. Calorie b. Energy associated with motion 2. chemical energy c. Energy associated with position 3. electrical energy d. Energy associated with the flow of electrical charge 4. endothermic e. Energy associated with potential chemical changes 5. exothermic f. 6. kilowatt-hour (kWh) Energy associated with random motions of atoms and molecules in matter g. Unit of energy equal to 1000 calories h. Unit of energy that appears on electricity bills i. Process that absorbs energy from the surroundings j. Process that emits energy to the surroundings 7. kinetic energy 8. law of conservation of energy 9. potential energy 10. thermal energy 15. Distinguish between potential and kinetic energy. 16. Classify each as having either kinetic energy or potential energy. a. batteries in a package b. a person that has climbed to the top of a tree c. a roller coaster in motion at the bottom of a hill d. air molecules in a balloon e. an arrow pulled back, ready to shoot f. a firework exploding 4 17. How many joules are in one calorie? How many calories are in one Calorie? 18. Convert 3.16 x 102 cal to joules. 19. If you were counting Calories, and consumed 2160 Calories in one day, what would this be in Joules? 20. Classify each of the following as either exothermic or endothermic changes: a. Freezing water c. Cooking an egg b. Boiling water d. Burning of propane Section 4 – Thermal Energy: (text pages 72-80) Need to be able to: Convert between Fahrenheit, Celsius, and Kelvin temperature scales. Relate energy, temperature change, and heat capacity. Explain specific heat capacity. Apply calorimetry when solving specific heat problems. Practice Problems: 21. Match the following terms with the phrases below: a. Temperature scale commonly used in the United States 11. calorimeter b. Temperature scale used by scientists that contains negative values 12. Celsius scale c. Temperature scale used by scientists that contains no negative values 14. heat capacity d. Quantity of heat energy required to raise the temperature of a given amount of substance by 1°C 16. specific heat capacity 13. Fahrenheit scale 15. Kelvin scale e. Amount of energy required to raise the temperature of 1 g of a substance by 1°C f. A tool used to determine the amount of heat an object contains by transferring that heat to water 5 22. Explain the energy of a sample of matter at absolute zero. 23. Convert 68°F to K. 24. Convert 36 K to °C. 25. The amount of energy needed to heat 2.00 g of carbon from 50.0°C to 80.0 °C is 42.6 J. What is the specific heat capacity of this sample of carbon? 26. The specific heat capacity of silver is 0.235 J/g °C. How many joules of energy are needed to warm 0.500 g of silver from 25.0 °C to 27.5 °C? 27. A 6.75 g sample of gold (specific heat capacity = 0.130 J/g °C) is heated using 50.6 J of energy. If the original temperature of the gold is 25.0 °C, what is its final temperature? 28. A 2.50 g sample of zinc is heated and then placed in a calorimeter containing 65.0 g of water. Temperature of water increases from 20.00 oC to 22.50 oC. The specific heat of zinc is 0.390 J/goC. What was the initial temperature of the zinc metal sample? 6