MLAB 2401: C C K B
advertisement

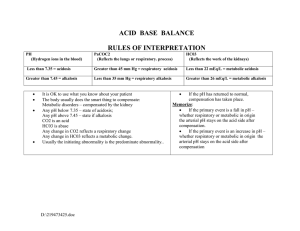
MLAB 2401: CLINICAL CHEMISTRY KERI BROPHY-MARTINEZ Disorders of Acid-Base Imbalance ACID-BASE IMBALANCES • • • • • pH< 7.35 = acidosis/acidemia pH> 7.45 = alkalosis/alkalemia The body responds to imbalances by compensation If balance is fully restored to 20:1 , it is termed complete If balance is still outside of normal limits it is termed partial COMPENSATION Respiratory compensation Occurs when underlying problem is metabolic See changes in pCO2 Body responds by hyper or hypoventilation Metabolic Compensation Occurs when underlying problem is respiratory See changes in bicarbonate concentration Body responds by activating renal mechanisms ACID-BASE IMBALANCE Four categories Metabolic Acidosis Metabolic Alkalosis Respiratory Acidosis Respiratory Alkalosis 5 METABOLIC VS RESPIRATORY Metabolic KIDNEY Effects base= bicarbonate Respiratory LUNGS Effects acid= carbonic acid METABOLIC ACIDOSIS Bicarbonate deficit : blood concentrations of bicarb drop below 22mEq/L Results in: pH drop Decrease in 20:1 ratio Causes of: Loss of bicarbonate through diarrhea or renal dysfunction Accumulation of acids (lactic acid or ketones) that exceed rate of elimination Failure of kidneys to excrete H+ 7 SYMPTOMS OF METABOLIC ACIDOSIS Headache, Rapid and deep breathing Lethargy Nausea, vomiting, diarrhea Coma Death 8 COMPENSATION FOR METABOLIC ACIDOSIS Respiratory Primary mechanism Increased ventilation CO2 blown off Renal Excretion of hydrogen ions if possible Reabsorption of bicarbonate 9 10 METABOLIC ALKALOSIS Bicarbonate excess - concentration in blood is greater than 26 mEq/L Results in: pH increase Causes of: Loss of acid-rich fluids Excess vomiting = loss of stomach acid Certain diuretics Addition of base to the body Excessive use of alkaline drugs Heavy ingestion of antacids Decrease of base elimination Endocrine disorders ( Cushing’s syndrome) 11 COMPENSATION FOR METABOLIC ALKALOSIS Respiratory Primary mechanism Hypoventilation Increased retention of CO2 Limited by hypoxia ( no oxygen) Alkalosis most commonly occurs with renal dysfunction, so can’t count on kidneys to excrete excess bicarbonate 12 SYMPTOMS OF METABOLIC ALKALOSIS Respiration slow and shallow Hyperactive reflexes ; tetany Often related to depletion of electrolytes Atrial tachycardia Dysrhythmias 13 14 RESPIRATORY ACIDOSIS • • • Increased carbonic acid as indicated by increased pCO2 Results in: decreased pH Causes of: – Problems within the respiratory system – Organs- lungs – Obstruction in the airway or restriction of gas exchange – Obstructive emphysema – Pulmonary edema/ pulmonary disease – Depression of respiratory center in brain that controls the breathing rate – Drugs – Stroke, Coma COMPENSATION FOR RESPIRATORY ACIDOSIS Kidneys Primary mechanism Eliminate hydrogen ions Retain bicarbonate ions SIGNS AND SYMPTOMS OF RESPIRATORY ACIDOSIS Breathlessness Restlessness Lethargy and disorientation Tremors, convulsions, coma Respiratory rate rapid, then gradually depressed Skin warm and flushed due to vasodilation caused by excess CO2 17 18 RESPIRATORY ALKALOSIS • Decrease carbonic acid indicated by decreased pCO2 Most common acid-base imbalance • Results in: increased pH • Causes of: • Hypoxemia • Stimulation of the Respiratory Center: • RESPIRATORY ALKALOSIS Hypoxemia Pulmonary disease Congestive heart disease Severe anemia High-altitude exposure Conditions that stimulate respiratory center: Acute anxiety Salicylate intoxication Cirrhosis Gram-negative sepsis Hyperventilation syndrome 20 COMPENSATION FOR RESPIRATORY ALKALOSIS • Kidneys Primary mechanism • Conserve hydrogen ion • Excretion of bicarbonate ion • 22 SUMMARY OF ACID-BASE DISORDERS PRIMARY ACID/BASE DISORDERS pCO2 pH HCO3 Base Excess Uncompensated acidosis N D D D Uncompensated alkalosis N I I I Partially compensated acidosis D D D D Partially compensated alkalosis I I I I N I/D I/D Compensated I/D Acidosis/alkalosis Disturbance Primary Abnormality Compensation Metabolic Excess endogenous Hyperventilation lowers Acidosis acid depletes pCO2, bicarbonate Kidney excretes excess H+ and forms more HCO3Respiratory Inefficient excretion Formation of excess Acidosis of CO2 by the HCO3- by kidney lungs Metabolic Alkalosis Excess plasma bicarbonate Kidneys excrete excess HCO3- and form less HCO3- and NH4, Lungs hypoventilate Respiratory Alkalosis Hyperventilation lowers pCO2 Increased excretion of bicarbonate by kidney Cause Renal failure Ketosis Increased lactic acid Diarrhea Chronic pulmonary Diseases (COPD), such as emphysema Acute problems, such as pneumonia, airway obstruction, drugs such as opiates, congestive heart failure Loss of gastric juice Chloride depletion Hypokalemia Increased corticosteroid Increased ingestion of antacids Hyperventilation, such as with severe anxiety, fever, head injuries Stimulation of resp. center by drugs Central nervous system diseases 26 REFERENCES Bishop, M., Fody, E., & Schoeff, l. (2010). Clinical Chemistry: Techniques, principles, Correlations. Baltimore: Wolters Kluwer Lippincott Williams & Wilkins. Carreiro-Lewandowski, E. (2008). Blood Gas Analysis and Interpretation. Denver, Colorado: Colorado Association for Continuing Medical Laboratory Education, Inc. Jarreau, P. (2005). Clinical Laboratory Science Review (3rd ed.). New Orleans, LA: LSU Health Science Center. Sunheimer, R., & Graves, L. (2010). Clinical Laboratory Chemistry. Upper Saddle River: Pearson . 27