23. Acid-base.doc
advertisement

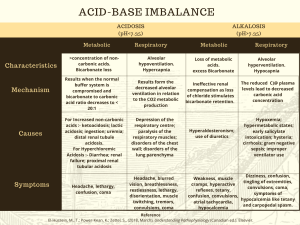
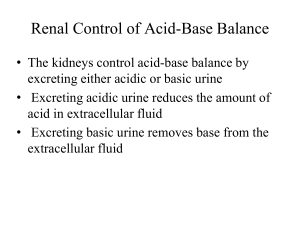
D’YOUVILLE COLLEGE BIOLOGY 108/508 - HUMAN ANATOMY & PHYSIOLOGY II LECTURE # 23 BODY FLUID PHYSIOLOGY ACID-BASE BALANCE 1. Acids, Bases and Buffers: a. Acids: yield hydrogen ions in solution; concentration: # of moles/liter; strength: degree of ionization (extent of H+ yield) • strong acids typically are mostly ionized yielding the maximum # of H+: HCl <- ---------> H+ + Cl• weak acids will be mostly non-ionized yielding small # of H+: HA <-------- --> H+ + A• respiratory acid: carbon dioxide forms weak acid in water (carbonic acid) • all other sources of hydrogen ions (e.g. lactic acid, hydrochloric acid, ketone bodies) are metabolic acids b. Bases: consume hydrogen ions from solution; the anion portion of an ionized acid is known as conjugate base c. Buffers: systems of weak acid + acid anion which react to offset changes in hydrogen ion concentration of a solution; increasing acidity causes buffer system to behave as a base; decreasing acidity causes buffer system to behave as an acid • Bicarbonate System: mostly associated with extracellular fluid; important because of linkage to respiratory and renal influences on pH H2O + CO2 <-- * --> H2CO3 <-------- ---> H+ + HCO3* = carbonic anhydrase enzyme • Protein System(s): highest concentration; mostly intracellular (e.g. hemoglobin oxygenation reaction) NPrCOOH <-- -->H+ + +H3NPrCOO-<-- --> 2H++ H2NPrCOO• Phosphate System: mostly intracellular, also important in renal tubules H3PO4 <-- -->H+ + H2PO4-<-- --> 2H+ + HPO42• Ammonia: mainly renal tubules NH4+ <-- --> H+ + NH3 2. Body Fluid pH: • intracellular fluid (cytoplasm) - normal pH = 7.0 • extracellular fluid (plasma, interstitial fluid) has normal pH = 7.4 • pHECF > 7.45 constitutes alkalosis; pHECF < 7.35 constitutes acidosis • alkalosis causes hyperactivity of nervous system (convulsions) • acidosis causes depression of nervous system (coma) • changes in PCO2 (high -> resp. acidosis; low -> respiratory alkalosis) • changes in other acids (high = metab. acidosis; low = metabolic alkalosis) • changes caused by HCO3- (low = metabolic acidosis; high = metabolic alkalosis) Bio 108/508 3. lec. 23 - p. 2 Defenses Against pH Changes: a. Buffers: systems interact to maintain pH between 7.35 - 7.45 b. Respiratory Adjustment: • hyperventilation blows off CO2 causing shift in bicarbonate system that consumes more H+ (alkalinizing effect, higher pH) • hypoventilation accumulates CO2 causing shift in bicarbonate system that elevates H+ (acidifying effect, lower pH) c. Renal Adjustment: kidneys secrete hydrogen ion (tubular secretion) in conjunction with bicarbonate absorption • Correcting Acidosis: kidneys secrete more H+ and retain more bicarbonate ion (bolstering buffer system) • Correcting Alkalosis: kidneys retain H+ and excrete more bicarbonate ion