MO diagram for homonuclear diatomic molecules O and F
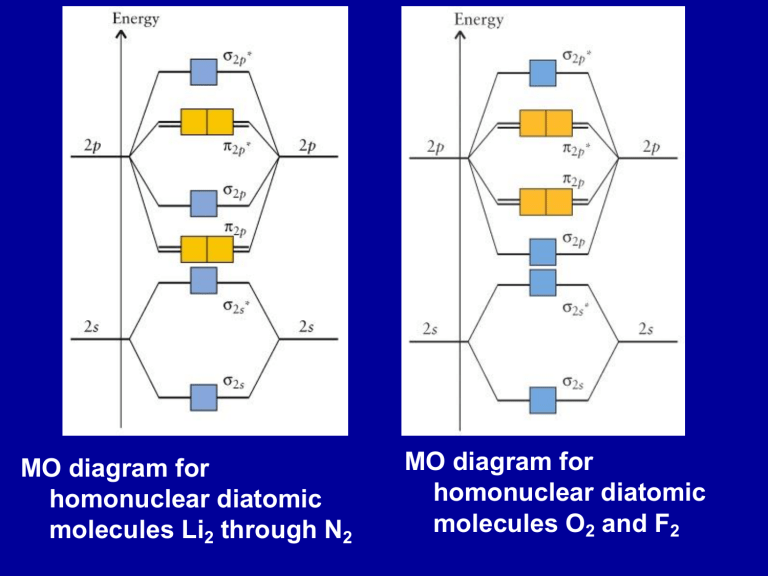
MO diagram for homonuclear diatomic molecules Li
2 through N
2
MO diagram for homonuclear diatomic molecules O
2 and F
2
N
2
Lewis diagram :N
N: each atom supplies 5 electrons; a total of 10 electrons must
N
2 be assigned
: s
2s
2 s
*
2s
2 p
2p
4 s
2p
2
Bond order for N
2
= 0.5 (8 - 2) = 3
O
2
O O
Lewis model
Molecular Orbital Theory
O
2
: s
2s
2 s
*
2s
2 s
2p
2 p
2p
4 p
*
2p
1 p
*
2p
1
Unpaired electrons
- paramagnetic
Bond order = 2
Agrees with Lewis diagram
Heteronuclear diatomics - electrons are unequally shared by the two atoms due to electronegativity differences.
y
= c
A y
A
+ c
B y
B
Where c
A and c
B are coefficients which indicate the contribution of each AO in the MO
In a homonuclear molecule: c
A
= c
B
= 1
MO energy-level diagram for a AB type molecule.
A is more electronegative than B
Energy levels of the more electronegative element is lower than the less electronegative element s
: higher contribution from A2s s *
: higher contribution from B2s
Lowest unoccupied MO
(LUMO)
Highest occupied MO
(HOMO)
CO - ten valence electrons
CO: s
2s
2 s
*
2s
2 p
2p
4 s
2p
2
Bond order = 0.5 (8 - 2) = 3
Polyatomics - same principles as diatomics except that the
MOs spread all over the atoms in the molecule
An electron pair helps to bind the whole molecule, not just a pair of atoms.
H
2
O: six atomic orbitals (one O2s, three O2p, and two H1s)
These six orbitals are used to build six molecular orbitals in which the degree of net bonding characters is related to the number of internuclear nodes.
Molecular Orbitals of H
2
O
http://www.whfreeman.com/chemicalprinciples/con_index.ht
m?03
http://www.shef.ac.uk/chemistry/orbitron/
Spectroscopy
Spectroscopy: interaction of light with matter
Average Bond energies (kJ/mol)
C-H: 413 C=C: 610 H-F: 565
H-H: 436 C
C: 835


