CHE 140 REVIEW CH #14-16
advertisement
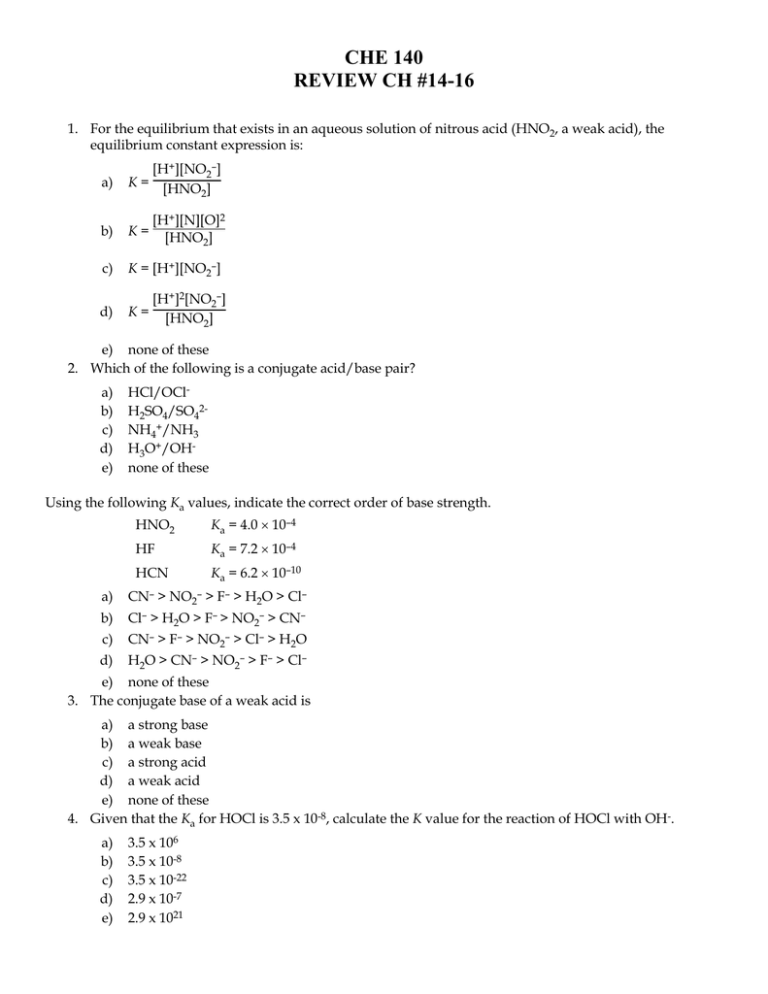
CHE 140 REVIEW CH #14-16 1. For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equilibrium constant expression is: a) [H+][NO2–] K = [HNO ] 2 b) K= c) K = [H+][NO2–] d) [H+]2[NO2–] K = [HNO ] 2 [H+][N][O]2 [HNO2] e) none of these 2. Which of the following is a conjugate acid/base pair? a) b) c) d) e) HCl/OClH2SO4/SO42NH4+/NH3 H3O+/OHnone of these Using the following Ka values, indicate the correct order of base strength. HNO2 Ka = 4.0 10–4 HF Ka = 7.2 10–4 HCN Ka = 6.2 10–10 a) CN– > NO2– > F– > H2O > Cl– b) Cl– > H2O > F– > NO2– > CN– c) CN– > F– > NO2– > Cl– > H2O d) H2O > CN– > NO2– > F– > Cl– e) none of these 3. The conjugate base of a weak acid is a) a strong base b) a weak base c) a strong acid d) a weak acid e) none of these 4. Given that the Ka for HOCl is 3.5 x 10-8, calculate the K value for the reaction of HOCl with OH-. a) b) c) d) e) 3.5 x 106 3.5 x 10-8 3.5 x 10-22 2.9 x 10-7 2.9 x 1021 5. Calculate the [H+] in a solution that has a pH of 11.70. a) 2.3 M b) 11.7 M c) 5.0 10–3 M d) 2.0 10–12 M e) none of these 6. The pH of a solution at 25°C in which [OH–] = 3.4 10–5 M is: a) 4.5 b) 10.5 c) 9.5 d) 6.3 e) none of these 7. Solid calcium hydroxide is dissolved in water until the pH of the solution is 10.94. The hydroxide ion concentration [OH–] of the solution is: a) 1.1 10–11 M b) 3.06 M c) 8.7 10–4 M d) 1.0 10–14 M e) none of these 8. As water is heated, its pH decreases. This means that a) the water is no longer neutral b) [H+] > [OH-] c) [OH-] > [H+] d) a and b are correct e) none of these 9. For nitrous acid, HNO2, Ka = 4.0 10–4. Calculate the pH of 0.25 M HNO2. a) 2.00 b) 2.30 c) 2.70 d) 3.70 e) none of these 10. Determine the molarity of a solution of the weak acid HClO2 (Ka = 1.10 10–2) if it has a pH of 1.25. a) 0.287 M b) 1.23 M c) 0.819 M d) 3.17 M e) 1.52 M 11. A solution of 2.5 M weak acid is 0.52% ionized. What is the Ka value of this acid? a) b) c) d) e) 6.8 x 10-5 1.1 x 10-5 0.11 1.3 x 10-2 none of these 12. A monoprotic weak acid when dissolved in water is 0.92% dissociated and produces a solution with a pH of 3.42. Calculate the Ka of the acid. a) 1.4 x 10-7 b) 2.8 x 10-3 c) 3.5 x 10-6 d) Need to know the initial concentration of the acid. e) None of these. 13. Which of the following reactions is associated with the definition of Kb? a) Zn(OH2)62+ [Zn(OH2)5OH]+ + H+ b) CN– + H+ HCN c) F– + H2O HF + OH– d) Cr3+ + 6H2O Cr(OH2)63+ e) none of these 14. Calculate the pH of a 0.10 M solution of Ca(OH)2. a) 13.30 b) 13.00 c) 0.20 d) 0.10 e) none of these 15. Determine the pH of a 0.060 M solution of H2SO4. The dissociation occurs in two steps. Ka1 is extremely large; Ka2 is 1.2 10–2. a) 1.40 b) 1.31 c) 1.22 d) 1.14 e) 1.00 16. The equilibrium constant for the reaction NH4+ + OH– is: a) 1 Kb(NH3) b) 1 Ka(NH4+) c) Kw Ka(NH4+) d) Kw Kb(NH3) e) Kb(NH3) Kw NH3 + H2O 17. Calculate the pH of the following aqueous solution: 0.57 M NaF (pKa for HF = 3.14) a) 5.55 b) 2.90 c) 8.45 d) 11.10 e) none of these 18. Calculate the [H+] in 1.0 M solution of Na2CO3 (for H2CO3, Ka1 = 4.3 10–7; Ka2 = 5.6 10–11). a) 7.5 10–6 M b) 6.6 10–4 M c) 1.3 10–2 M d) 7.5 10–13 M e) none of these 19. Which of the following is the strongest base? (Kb for NH3 is 1.8 10–5, Ka2 for H2SO4 is 1.2 10–2, Ka3 for H3PO4 is 4.8 10–13) NH3, HSO4–, PO43–, or NO3– a) NH3 b) HSO4– c) NO3– d) PO43– e) Two of these are equally strong. 20. The [H3O+] of a 0.15 M solution of NH4Cl in H2O at 25°C is (Kb for NH3 = 1.8 10–5): a) 3.6 10–3 M b) 4.9 10–5 M c) 9.1 10–6 M d) 8.2 10–10 M 21. If Ka for HCN is 6.2 10–10, what is Kb for CN–? Note: CN– + H2O HCN + OH– Kb = [HCN][OH–] [CN–] a) 6.2 10–24 b) 6.2 104 c) 1.6 10–5 d) 1.6 1023 e) none of these 22. The hydrogen halides (HF, HCl, HBr, and HI) are all polar molecules. The strength of the acid each forms in water is based on which of the following? a) b) c) d) e) the polarity of the molecule the size of the molecule the strength of the bond two of these none of these 23. Which factor listed below is most important in determining the strength of an oxyacid? a) the size of the molecule b) the ability of the molecule to change atomic orientation c) the identity of the central atom in the molecule d) the number of oxygen atoms present in the molecule e) none of these 24. Which of the following would produce a basic aqueous solution? a) P4O10 b) KCl c) CO2 d) NH4Cl e) none of these 25. Calculate the pH of a 0.005 M solution of potassium oxide, K2O. a) 12.0 b) 11.7 c) 7.0 d) 2.3 e) 2.0 26. Which of the species below, when dissolved in H2O, will not produce a basic solution? a) SO2 b) NH3 c) BaO d) Ba(OH)2 e) none of these 27. HA and HB are both weak acids in water, and HA is a stronger acid than HB. Which of the following statements is correct? a) A- is a stronger base than B-, which is a stronger base than H2O, which is a stronger base than Cl-. b) B- is a stronger base than A-, which is a stronger base than H2O, which is a stronger base than Cl-. c) B- is a stronger base than A-, which is a stronger base than Cl-, which is a stronger base than H2O. d) Cl- is a stronger base than A-, which is a stronger base than B-, which is a stronger base than H2O. e) None of these (a-d) is correct. 28. You have solutions of 0.200 M HNO2 and 0.200 M KNO2 (Ka for HNO2 = 4.00 10–4). A buffer of pH 3.000 is needed. What volumes of HNO2 and KNO2 are required to make 1 liter of buffered solution? a) b) c) d) e) 500 mL of each 286 mL HNO2; 714 mL KNO2 413 mL HNO2; 587 mL KNO2 714 mL HNO2; 286 mL KNO2 587 mL HNO2; 413 mL KNO2 29. 15.0 mL of 0.50 M HCl is added to a 100.-mL sample of 0.200 M HNO2 (Ka for HNO2 = 4.0 10–14). What is the equilibrium concentration of NO2– ions? a) 1.1 10–13 M b) 6.5 10–12 M c) 7.5 10–14 M d) 1.7 10–11 M e) none of these 30. A solution contains 0.250 M HA (Ka = 1.0 x 10-6) and 0.45 M NaA. What is the pH after 0.10 mole of HCl is added to 1.00 L of this solution? a) 3.17 b) 3.23 c) 6.00 d) 10.77 e) 10.83 31. If one adds 0.30 liters of 0.020 M KOH to the solution what will be the change in pH? a) 0.02 b) 0.20 c) 0.41 d) 0.53 e) none of these 32. Calculate the pH of a solution that is 2.00 M HF, 1.00 M NaOH, and 0.500 M NaF. (Ka is 7.2 x 10-4) a) 3.14 b) 3.32 c) 3.02 d) 2.84 e) none of these 33. Given 100.0 mL of a buffer that is 0.50 M in HOCl and 0.40 M in NaOCl, what is the pH after 10.0 mL of 1.0 M NaOH has been added? (Ka for HOCl = 3.5 10–8) a) 6.45 b) 6.64 c) 7.36 d) 7.45 e) 7.55 34. One milliliter (1.00 mL) of acid taken from a lead storage battery is pipetted into a flask. Water and phenolphthalein indicator are added, and the solution is titrated with 0.50 M NaOH until a pink color appears; 12.0 mL are required. The number of grams of H2SO4 (formula weight = 98) present in one liter of the battery acid is (to within 5%): a) b) c) d) e) 240 290 480 580 750 35. You are given 5.00 mL of an H2SO4 solution of unknown concentration. You divide the 5.00-mL sample into five 1.00-mL samples and titrate each separately with 0.1000 M NaOH. In each titration the H2SO4 is completely neutralized. The average volume of NaOH solution used to reach the endpoint is 15.3 mL. What was the concentration of H2SO4 in the 5.00-mL sample? a) 1.53 M b) 0.306 M c) 0.765 M d) 1.53 10–3 M e) 3.06 M 36. If 25.0 mL of 0.451 M NaOH solution is titrated with 0.253 M H2SO4, the flask at the endpoint will contain (besides the indicator phenolphthalein) as the principal components: a) sodium hydroxide, sulfuric acid, and water b) dissolved sodium sulfate and water c) sodium hydroxide, sodium sulfate, and water d) dissolved sodium sulfate, sulfuric acid, and water e) precipitated sodium sulfate and water 37. Consider the titration of 500.0 mL of 0.200 M NaOH with 0.800 M HCl. How many milliliters of 0.800 M HCl must be added to reach a pH of 13.000? a) 55.6 mL b) 24.6 mL c) 18.5 mL d) 12.9 mL e) 4.32 mL 38. Consider the titration of 100.0 mL of 0.250 M aniline (Kb = 3.8 10–10) with 0.500 M HCl. For calculating the volume of HCl required to reach a pH of 8.0, which of the following expressions is correct? (x = volume in mL of HCl required to reach a pH of 8.0) a) 0.5x – (100)(0.25) = [aniline] 100 + x b) [H+] = x c) 0.5x 100 + x = [aniline] d) 25 – 0.5x –6 100 + x – 10 = [aniline] e) none of these 39. Calculate the pH at the equivalence point for the titration of 1.0 M ethylamine, C2H5NH2, by 1.0 M perchloric acid, HClO4. (pKb for C2H5NH2 = 3.25) a) b) c) d) e) 6.05 2.24 5.53 2.09 5.38 40. In the titration of a weak acid, HA, with a sodium hydroxide solution the stoichiometric point occurs at pH = 9.5. Which of the following weak acid indicators would be best suited to mark the endpoint of this titration? a) HB, Ka = 10–11 b) HC, Ka = 10–13 c) HD, Ka = 10–9 d) HE, Ka = 10–7 41. When 0.10 mol of H+ ions is added to the solution what is the pH? a) 8.40 b) 9.63 c) 9.41 d) 9.18 e) 9.72 42. Find the solubility (in mol/L) of lead(II) chloride (PbCl2) at 25C. Ksp = 1.6 10–5 a) 1.6 10–2 b) 0.020 c) 7.1 10–5 d) 2.1 e) 9.3 10–3 43. Methyl orange is an indicator with a Ka of 1 10–4. Its acid form, HIn, is red, while its base form, In–, is yellow. At pH 6.0, the indicator will be a) red. b) orange. c) yellow. d) blue. e) not enough information 44. Solubility Products (Ksp) BaSO4 1.5 10–9 CoS 5.0 10–22 PbSO4 1.3 10–8 AgBr 5.0 10–13 45. Which of the following compounds is the most soluble (in moles/liter)? a) BaSO4 b) CoS c) PbSO4 d) AgBr e) BaCO3 46. How many moles of Fe(OH)2 [Ksp = 1.8 10–15] will dissolve in one liter of water buffered at pH = 12.00? a) b) c) d) e) 1.8 10–11 1.8 10–9 8.0 10–6 5.0 10–12 4.0 10–8 47. You have a solution consisting of 0.10 M Cl– and 0.10 M CrO42–. You add 0.10 M silver nitrate dropwise to this solution. Given that the Ksp for Ag2CrO4 is 9.0 10–12, and that for AgCl is 1.6 10–10, which of the following will precipitate first? a) silver chloride b) silver chromate c) silver nitrate d) cannot be determined by the information given e) none of these 48. The Ksp for PbF2 is 4.0 10–8. If a 0.050 M NaF solution is saturated with PbF2, what is the [Pb2+] in solution? a) 3.8 10–3 M b) 3.6 10–4 M c) 5.4 10–7 M d) 1.6 10–5 M e) 2.7 10–8 M 49. Which of the following salts shows the lowest solubility in water? (Ksp values: Ag2S = 1.6 10–49; Bi2S3 = 1.0 10–72; HgS = 1.6 10–54; Mg(OH)2 = 8.9 10–12; MnS = 2.3 10–13) a) Bi2S3 b) Ag2S c) MnS d) HgS e) Mg(OH)2 50. Which of the following compounds has the lowest solubility in mol/L in water at 25C? a) Ag3PO4 Ksp = 1.8 10–18 b) Sn(OH)2 Ksp = 5 10–26 c) CdS Ksp = 3.6 10–29 d) CaSO4 Ksp = 6.1 10–5 e) Al(OH)3 Ksp = 2 10–33 51. A 100.-mL sample of solution contains 10.0 mmol of Ca2+ ion. How many mmol of solid Na2SO4 must be added in order to cause precipitation of 99.9% of the calcium as CaSO4? The Ksp of CaSO4 is 6.1 10–5. Assume the volume remains constant. a) 17.4 b) 10.0 c) 61.0 d) 71.0 e) 2.00 52. Will precipitation occur? a) b) c) d) e) Yes No Maybe, it depends on the temperature. Maybe, it depends on the limiting reagent concentration. None of these. 53. What is the limiting reagent in the formation of the lead chloride? a) Pb2+ b) Cl– c) (NO3)– d) PbCl2 e) Pb(NO3)2 54. Silver acetate (AgC2H3O2) is a sparingly soluble salt with Ksp = 1.9 10–3. Consider a saturated solution in equilibrium with the solid salt. Compare the effects on the solubility of adding to the solution either the acid HNO3 or the base NH3. a) Either substance would decrease the solubility. b) NH3 would increase the solubility, but HNO3 would decrease it. c) NH3 would increase the solubility, but HNO3 would have virtually no effect. d) Either substance would increase the solubility. e) NH3 would decrease the solubility, but HNO3 would increase it. 55. The Ksp for Mn(OH)2 is 2.0 10–13. At what pH will Mn(OH)2 begin to precipitate from a solution in which the initial concentration of Mn2+ is 0.10 M? a) 6.47 b) 13.3 c) 5.85 d) 7.03 e) 8.15 56. Which of the following solid salts should be more soluble in 1.0 M NH3 than in water? a) Na2CO3 b) KCl c) AgBr d) KNO3 e) none of these 57. Given the following values of equilibrium constants: Cu(OH)2(s) Cu2+(aq) + 2OH–(aq) Ksp = 1.6 19–19 Cu(NH3)42+ Cu2+(aq) + 4NH3(aq) K = 1.0 10–13 What is the value of the equilibrium constant for the reaction: Cu(OH)2(s) + 4NH3(aq) Cu(NH3)42+(aq) + 2OH–(aq) a) 1.6 10–19 b) 6.2 1031 c) 1.6 10–6 d) 1.6 10–32 e) 1.0 1013 58. In which reaction is S° expected to be positive? a) b) c) d) e) I2(g) I2(s) H2O(1) H2O(s) CH3OH(g) + (3/2)O2(g) CO2(g) + 2H2O(l) 2O2(g) + 2SO(g) 2SO3(g) none of these 59. Which statement is true? a) b) c) All real processes are irreversible. A thermodynamically reversible process takes place infinitely fast. In a reversible process, the state functions of the system are always much greater than those of the surroundings. d) There is always more heat given off to the surroundings in a reversible process than in an unharnessed one. e) All statements (a–d) are true. 60. A mixture of hydrogen and chlorine remains unreacted until it is exposed to ultraviolet light from a burning magnesium strip. Then the following reaction occurs very rapidly: H2(g) + Cl2(g) 2HCl(g) G = –45.54 kJ H = –44.12 kJ S = –4.76 J/K Select the statement below that best explains this behavior. a) The reactants are thermodynamically more stable than the products. b) The reaction has a small equilibrium constant. c) The ultraviolet light raises the temperature of the system and makes the reaction more favorable. d) The negative value for S slows down the reaction. e) The reaction is spontaneous, but the reactants are kinetically stable. 61. Ten identical coins are shaken vigorously in a cup and then poured out onto a table top. Which of the following distributions has the highest probability of occurrence? (T = Tails, H = Heads) a) T10H0 b) T8H2 c) T7H3 d) T5H5 e) T4H6 62. A chemical reaction is most likely to be spontaneous if it is accompanied by a) increasing energy and increasing entropy. b) lowering energy and increasing entropy. c) increasing energy and decreasing entropy. d) lowering energy and decreasing entropy. e) None of these (a-d) 63. Which of the following statements is (are) always true? I. In order for a process to be spontaneous, the entropy of the universe must increase. II. A system cannot have both energy disorder and positional disorder. G III. Suniv = T IV. a) b) c) d) e) S° is zero for elements in their standard states. I I, IV I, III, IV II, IV II 64. Which of the following statements is always true for a spontaneous process? I. Ssys > 0 II. Ssurr > 0 III. Suniv > 0 IV. Gsys > 0 a) I b) III c) IV d) I and III e) III and IV 65. Assume that the enthalpy of fusion of ice is 6020 J/mol and does not vary appreciably over the temperature range 270–290 K. If one mole of ice at 0°C is melted by heat supplied from surroundings at 280. K, what is the entropy change in the surroundings, in J/K? a) +22.0 b) +21.5 c) 0.0 d) –21.5 e) –22.0 66. The heat of vaporization for 1.0 mole of water at 100.°C and 1.0 atm is 40.6 kJ/mol. Calculate S for the process H2O(l) H2O(g) at 100.°C. a) b) c) d) e) 67. Ssurr 109 J/K mol –109 J/K mol 406 J/K mol –406 J/K mol none of these is _______ for exothermic reactions and ______ for endothermic reactions. a) favorable, unfavorable b) unfavorable, favorable c) favorable, favorable d) unfavorable, unfavorable e) cannot tell 68. For the process S8 (rhombic) S8 (monoclinic) at 110°C, H = 3.21 kJ/mol and S = 8.70 J/K mol (at 110°C). Which of the following is correct? a) This reaction is spontaneous at 110°C (S8 (monoclinic) is stable). b) This reaction is spontaneous at 110°C (S8 (rhombic) is stable). c) This reaction is nonspontaneous at 110°C (S8 (rhombic) is stable). d) This reaction is nonspontaneous at 110°C (S8 (monoclinic) is stable). e) Need more data. 69. Given the following data (Hf, S°, respectively) for N2O4 (l) –20. kJ/mol, 209 J/K mol, and N2O4(g) 10. kJ/mol, 304.0 J/K mol. Above what temperature (in °C) is the vaporization of N2O4 liquid spontaneous? a) b) c) d) e) Above -178°C. Above -230°C. Above 3°C. Above 30.°C. Above 43°C. 70. Elemental sulfur exists in two crystalline forms, rhombic and monoclinic. From the following data, calculate the equilibrium temperature at which monoclinic sulfur and rhombic sulfur are in equilibrium. Hf° (kJ/mol) S (rhombic) S (monoclinic) 0 0.30 S° (J/K mol) 31.88 32.55 a) +450 K b) +210 K c) –210 K d) –450 K e) 0 K 71. The reaction 2H2O(g) 2H2(g) + O2(g) has a positive value of G°. Which of the following statements must be true? a) b) The reaction is slow. The reaction will not occur. [When H2O(g) is introduced into a flask, no O2 or H2 will form even over a long period of time.] c) The reaction is exothermic. d) The equilibrium lies far to the right. e) None of these is true. 72. The standard free energy of formation of AgCl(s) is –110 kJ/mol. G° for the reaction 2AgCl(s) 2Ag(s) + Cl2(g) is: a) 110 kJ b) 220 kJ c) –110 kJ d) –220 kJ e) none of these 73. Given the following free energies of formation: G°f C2H2(g) 209.2 kJ/mol C2H6(g) –32.9 kJ/mol calculate Kp at 298 K for C2H2(g) + 2H2(g) C2H6(g) a) 9.07 10–1 b) 97.2 c) 1.24 1031 d) 2.72 1042 e) None of these is within a factor of 10 of the correct answer. 74. The acid dissociation constant for a weak acid HX at 25°C is 1.60 10–8. Calculate the free energy of formation for X–(aq) at 25°C. The standard free energies of HX(aq) and H+(aq) at 25°C are –211.5 kJ/mol and 0, respectively. a) b) c) d) e) –12.5 kJ/mol 255 kJ/mol 0 –167 kJ/mol 322 kJ/mol 75. Given that G°f for NH3 = –16.67 kJ/mol, calculate the equilibrium constant for the following reaction at 298 K: N2(g) + 3H2(g) 2NH3(g) a) 6.98 105 b) 8.36 102 c) 8.36 10–2 d) 1.20 10–3 e) 1.42 10–6 76. Calculate G° for the reaction at 25°C. a) –135 kJ b) 98.7 kJ c) –25.2 kJ d) –11.2 kJ e) 0 77. At 699 K, G° = –23.25 kJ for the reaction H2(g) + I2(g) 2HI(g). Calculate G for this reaction if the reagents are both supplied at 10.0 atm pressure and the product is at 1.00 atm pressure. a) –3.5 kJ b) –36.6 kJ c) +36.6 kJ d) –50.0 kJ e) +50.0 kJ 78. Consider the following hypothetical reaction (at 310. K). Standard free energies in kJ/mol are given in parentheses. A (–32.2) B (207.8) + C (–237.0) G° = ? What is the value of the equilibrium constant for the reaction at 310. K? a) b) c) d) e) 0.31 1.9 1.0 2.2 1010 4.5 10–11 79. The equilibrium constant of a certain reaction was measured at various temperatures to give the plot shown above. What is S° for the reaction in J/mol K? a) b) c) d) e) 0.20 3.0 25 –50. –8.3 103 80. Consider a weak acid, HX. If a 0.10 M solution of HX has a pH of 5.83 at 25°C, what is G° for the acid’s dissociation reaction at 25°C? a) –61 kJ b) –30. kJ c) 0 d) 30. kJ e) 61 kJ 81. For a certain process, at 300. K, G = –10.0 kJ and H = –7.0 kJ. If the process is carried out reversibly, the amount of useful work that can be performed is a) –23.0 kJ b) –7.0 kJ c) –3.0 kJ d) –10.0 kJ e) –17.0 kJ 82. Determine the sign of S, Ssurr, and Suniv for the system (ice/saltwater) in Vessel #2. Vessel #2 S Ssurr Suniv a) 0 0 0 b) + 0 c) + + + d) + + e) + 0 + 83. Which of the following is true? a) By spontaneous we mean that the reaction or process will always proceed to the right (as written) even if very slowly. Increasing the temperature may speed up the reaction, but it does not affect the spontaneity of the reaction. b) By spontaneous we mean that the reaction or process will always proceed to the left (as written) even if very slowly. Increasing the temperature may speed up the reaction, but it does not affect the spontaneity of the reaction. c) By spontaneous we mean that the reaction or process will always proceed to the left (as written) even if very slowly. Increasing the temperature may speed up the reaction and it generally affects the spontaneity of the reaction. d) By spontaneous we mean that the reaction or process will always proceed to the right (as written) even if very slowly. Increasing the temperature may speed up the reaction, and it generally affects the spontaneity of the reaction. e) None of the above is true.