Topic 3
advertisement

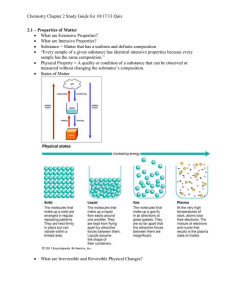
Topic 3 Table of Contents Topic 3 Topic 3: Matter—Properties and Change Basic Concepts Additional Concepts Matter—Properties and Change: Basic Concepts Topic 3 • • • • Properties of Matter—Substances Matter that has a uniform and unchanging composition is called a substance, also known as a pure substance. Table salt is a substance. Another example of a pure substance is water. Water is always composed of hydrogen and oxygen. Matter—Properties and Change: Basic Concepts Topic 3 Properties of Matter—Substances • Seawater, on the other hand, is not a substance because samples taken from different locations will probably have differing compositions. • That is, they will contain differing amounts of water, salts, and other dissolved substances. Matter—Properties and Change: Basic Concepts Topic 3 Physical Properties of Matter • A physical property is a characteristic that can be observed or measured without changing the sample’s composition. Physical properties describe pure substances, too. • Because substances have uniform and unchanging compositions, they have consistent and unchanging physical properties as well. Matter—Properties and Change: Basic Concepts Topic 3 Physical Properties of Matter Click box to view movie clip. Matter—Properties and Change: Basic Concepts Topic 3 Physical Properties of Matter • density, • taste, • color, • hardness, • odor, • melting point, • and boiling point are common physical properties that scientists record as identifying characteristics of a substance. Matter—Properties and Change: Basic Concepts Topic 3 Extensive and Intensive Properties • Physical properties can be further described as being one of two types. • Extensive properties are dependent upon the amount of substance present. For example, mass, which depends on the amount of substance there is, is an extensive property. • Length and volume are also extensive properties. Matter—Properties and Change: Basic Concepts Topic 3 Extensive and Intensive Properties • Density, on the other hand, is an example of an intensive property of matter. • Intensive properties are independent of the amount of substance present. • For example, density of a substance (at constant temperature and pressure) is the same no matter how much substance is present. Matter—Properties and Change: Basic Concepts Topic 3 Chemical Properties of Matter • The ability of a substance to combine with or change into one or more other substances is called a chemical property. • The ability of iron to form rust when combined with air is an example of a chemical property of iron • Similarly, the inability of a substance to change into another substance is also a chemical property. Matter—Properties and Change: Basic Concepts Topic 3 Chemical Properties of Matter • For example, when iron is placed in nitrogen gas at room temperature, no chemical change occurs. • The fact that iron does not undergo a change in the presence of nitrogen is another chemical property of iron. Matter—Properties and Change: Basic Concepts Topic 3 Observing Properties of Matter • Every substance has its own unique set of physical and chemical properties. • Observations of properties may vary depending on the conditions of the immediate environment. • It is important to state the specific conditions in which observations are made because both chemical and physical properties depend on temperature and pressure. Matter—Properties and Change: Basic Concepts Topic 3 States of Matter • In fact, all matter that exists on Earth can be classified as one of these physical forms called states of matter. • Scientists recognize a fourth state of matter called plasma, but it does not occur naturally on Earth except in the form of lightning bolts. Matter—Properties and Change: Basic Concepts Topic 3 States of Matter • The physical state of a substance is a physical property of that substance. • Each of the three common states of matter can be distinguished by the way it fills a container. Matter—Properties and Change: Basic Concepts Topic 3 Solids • A solid is a form of matter that has its own definite shape and volume. • Wood, iron, paper, and sugar are examples of solids. Matter—Properties and Change: Basic Concepts Topic 3 Solids • The particles of matter in a solid are very tightly packed; when heated, a solid expands, but only slightly. • Because its shape is definite, a solid may not conform to the shape of the container in which it is placed. Matter—Properties and Change: Basic Concepts Topic 3 Liquids • A liquid is a form of matter that flows, has constant volume, and takes the shape of its container. • Common examples of liquids include water, blood, and mercury. • The particles in a liquid are not rigidly held in place and are less closely packed than are the particles in a solid: liquid particles are able to move past each other. Matter—Properties and Change: Basic Concepts Topic 3 Liquids • This allows a liquid to flow and take the shape of its container, although it may not completely fill the container. Matter—Properties and Change: Basic Concepts Topic 3 Liquids • Because of the way the particles of a liquid are packed, liquids are virtually incompressible. Like solids, liquids tend to expand when heated. Matter—Properties and Change: Basic Concepts Topic 3 Gases • A gas is a form of matter that flows to conform to the shape of its container and fills the entire volume of its container. • Compared to solids and liquids, the particles of gases are very far apart. • Because of the significant amount of space between particles, gases are easily compressed. Matter—Properties and Change: Basic Concepts Topic 3 Gases • The word gas refers to a substance that is naturally in the gaseous state at room temperature. • The word vapor refers to the gaseous state of a substance that is a solid or a liquid at room temperature. • For example, steam is a vapor because at room temperature water exists as a liquid. Matter—Properties and Change: Basic Concepts Topic 3 Gases • The fact that substances can change form, as in the example of water changing to steam, is another important concept in chemistry • If you review what you just learned about physical properties of substances, you can see that because the particular form of a substance is a physical property, changing the form introduces or adds another physical property to its list of characteristics. Matter—Properties and Change: Basic Concepts Topic 3 Gases • In fact, resources that provide tables of physical and chemical properties of substances, such as the CRC Handbook of Chemistry and Physics, generally include the physical properties of substances in all of the states in which they can exist. Matter—Properties and Change: Basic Concepts Topic 3 Physical Changes • A substance often undergoes changes that result in a dramatically different appearance yet leave the composition of the substance unchanged. • An example is the crumpling of a sheet of aluminum foil. Matter—Properties and Change: Basic Concepts Topic 3 Physical Changes • While the foil goes from a smooth, flat, mirrorlike sheet to a round, compact ball, the actual composition of the foil is unchanged— it is still aluminum • Changes such as this, which alter a substance without changing its composition, are known as physical changes. Matter—Properties and Change: Basic Concepts Topic 3 Physical Changes • As with other physical properties, the state of matter depends on the temperature and pressure of the surroundings. • As temperature and pressure change, most substances undergo a change from one state (or phase) to another. Matter—Properties and Change: Basic Concepts Topic 3 Physical Changes • Melting and formation of a gas are both physical changes and phase changes. • When you encounter terms such as boil, freeze, condense, vaporize, or melt in your study of chemistry, the meaning generally refers to a phase change in matter. Matter—Properties and Change: Basic Concepts Topic 3 Chemical Changes • Chemical properties relate to the ability of a substance to combine with or change into one or more substances. • A process that involves one or more substances changing into new substances is called a chemical change, which is commonly referred to as a chemical reaction. Matter—Properties and Change: Basic Concepts Topic 3 Chemical Changes • The new substances formed in the reaction have different compositions and different properties from the substances present before the reaction occurred. Matter—Properties and Change: Basic Concepts Topic 3 Chemical Changes • When a freshly exposed iron surface is left in contact with air, it slowly changes into a new substance, namely, the rust. • The iron reacts with oxygen in the air to form a new substance, rust. Matter—Properties and Change: Basic Concepts Topic 3 Chemical Changes • Rust is a chemical combination of iron and oxygen. • In chemical reactions, the starting substances are called reactants and the new substances that are formed are called products. Matter—Properties and Change: Basic Concepts Topic 3 Chemical Changes • Thus iron and oxygen are reactants and rust is a product. • When you encounter terms such as explode, rust, oxidize, corrode, tarnish, ferment, burn, or rot, the meaning generally refers to a chemical reaction in which reactant substances produce different product substances. Matter—Properties and Change: Basic Concepts Topic 3 Conservation of Mass • By carefully measuring mass before and after many chemical reactions, it was observed that, although chemical changes occurred, the total mass involved in the reaction remained constant. • The constancy of mass in chemical reactions was observed so often that scientists assumed the phenomenon must be true for all reactions. Matter—Properties and Change: Basic Concepts Topic 3 Conservation of Mass Click box to view movie clip. Matter—Properties and Change: Basic Concepts Topic 3 Conservation of Mass • They summarized this observation in a scientific law. • The law of conservation of mass states that mass is neither created nor destroyed during a chemical reaction—it is conserved. • This law was one of the great achievements of eighteenth-century science. Matter—Properties and Change: Basic Concepts Topic 3 Conservation of Mass • The equation form of the law of conservation of mass is: Matter—Properties and Change: Basic Concepts Topic 3 Conservation of Mass • Lavoisier’s experimental decomposition of mercury(II) oxide is one proof of the law of conservation of mass. • Although a chemical reaction is obvious, matter was neither created nor destroyed. • The law of conservation of mass is one of the most fundamental concepts of chemistry. Basic Assessment Questions Topic 3 Question 1 Identify each of the following as a property of a solid, liquid, or gas. Some answers will include more that one state of matter. Basic Assessment Questions Topic 3 A. flows and takes the shape of a container B. compressible C. made of particles held in a specific arrangement D. has definite volume E. always occupies the entire space of its container F. has a definite volume but flows Basic Assessment Questions Topic 3 Answers A. flows and takes the shape of a container liquid, gas B. compressible gas C. made of particles held in a specific arrangement solid Basic Assessment Questions Topic 3 Answers D. has definite volume solid, liquid E. always occupies the entire space of its container gas F. has a definite volume but flows liquid Basic Assessment Questions Topic 3 Question 2 Identify each of the following as an example of a chemical change or a physical change. A. Moisture in the air forms beads of water on a cold windowpane. B. An electric current changes water into hydrogen and oxygen. C. Yeast cells in bread dough make carbon dioxide an ethanol from sugar. Basic Assessment Questions Topic 3 Answers A. Moisture in the air forms beads of water on a cold windowpane. Physical B. An electric current changes water into hydrogen and oxygen. Chemical C. Moisture in the air forms beads of water on a cold windowpane. Chemical Basic Assessment Questions Topic 3 Question 3 A reaction between sodium hydroxide and hydrogen chloride gas produces sodium chloride and water. A reaction of 22.85 g of sodium hydroxide with 20.82 g of hydrogen chloride gives off 10.29 g of water. What mass of sodium chloride is formed in the reaction? Basic Assessment Questions Topic 3 Answer 33.38 g sodium chloride Matter—Properties and Change: Additional Concepts Topic 3 Additional Concepts Matter—Properties and Change: Additional Concepts Topic 3 Mixtures • A mixture is a combination of two or more pure substances in which each pure substance retains its individual chemical properties. • The composition of mixtures is variable, and the number of mixtures that can be created by combining substances is infinite. Matter—Properties and Change: Additional Concepts Topic 3 Mixtures • Although much of the focus of chemistry is the behavior of substances, it is important to remember that most everyday matter occurs as mixtures. • Substances tend naturally to mix; it is difficult to keep things pure. Matter—Properties and Change: Additional Concepts Topic 3 Mixtures • Two mixtures, sand and water, and table salt and water, are shown. • You know water to be a colorless liquid. Matter—Properties and Change: Additional Concepts Topic 3 Mixtures • Sand is a grainy solid that does not dissolve in water. • When sand and water are mixed, the two substances are in contact, yet each substance retains its properties. Matter—Properties and Change: Additional Concepts Topic 3 Mixtures • The sand and water have not reacted. • Just by looking at the sand–water mixture in beaker A, it is easy to see each separate substance. Matter—Properties and Change: Additional Concepts Topic 3 Mixtures • Some mixtures, however, may not look like mixtures at all. • The mixture of table salt and water in the beaker labeled B is colorless and appears the same as pure water. • How can you determine if it is a mixture? Matter—Properties and Change: Additional Concepts Topic 3 Mixtures • If you were to boil away the water, you would see a white residue. That residue is the salt. Thus, the colorless mixture actually contained two separate substances. Matter—Properties and Change: Additional Concepts Topic 3 Mixtures • The salt and the water physically mixed but did not react and were separated by the physical method of boiling. Matter—Properties and Change: Additional Concepts Topic 3 Types of Mixtures • Mixtures themselves are classified as either heterogeneous or homogeneous. • A heterogeneous mixture is one that does not blend smoothly throughout and in which the individual substances remain distinct. • The sand and water mixture is an example of a heterogeneous mixture. Matter—Properties and Change: Additional Concepts Topic 3 Types of Mixtures • Suppose you draw a drop from the top of the mixture using an eyedropper. • The drop would be almost completely water. Matter—Properties and Change: Additional Concepts Topic 3 Types of Mixtures • If you draw a second drop from the bottom of the mixture, that drop would contain mostly sand. • Thus the composition of the sand–water mixture is not uniform—the substances have not blended smoothly and the two substances of the mixture (sand on the bottom and water on the top) remain distinct. Matter—Properties and Change: Additional Concepts Topic 3 Types of Mixtures • A homogeneous mixture has constant composition throughout; it always has a single phase. • Let’s examine the salt–water mixture using the eyedropper. • A drop of the mixture from the top of the beaker has the same composition as a drop from the bottom of the beaker. In fact, every drop of the mixture contains the same relative amounts of salt and water. Matter—Properties and Change: Additional Concepts Topic 3 Types of Mixtures • Homogeneous mixtures are also referred to as solutions. Matter—Properties and Change: Additional Concepts Topic 3 Types of Mixtures • You are probably most familiar with solutions in a liquid form, such as cough suppressant medicine and lemonade, but solutions may contain solids, liquids, or gases. Matter—Properties and Change: Additional Concepts Topic 3 Types of Mixtures • The solid–solid solution known as steel is called an alloy. • An alloy is a homogeneous mixture of metals, or a mixture of a metal and a nonmetal in which the metal substance is the major component. Matter—Properties and Change: Additional Concepts Topic 3 Separating Mixtures • Most matter exists naturally as mixtures. • Because the substances in a mixture are physically combined, the processes used to separate a mixture are physical processes that are based on the difference in physical properties of the substances. • Sometimes it is very easy to separate a mixture; separating a mixture of pennies and nickels is not a difficult task. Matter—Properties and Change: Additional Concepts Topic 3 Separating Mixtures • Heterogeneous mixtures composed of solids and liquids are easily separated by filtration. • Filtration is a technique that uses a porous barrier to separate a solid from a liquid. Matter—Properties and Change: Additional Concepts Topic 3 Separating Mixtures • Most homogeneous mixtures can be separated by distillation • Distillation is a separation technique that is based on differences in the boiling points of the substances involved. Matter—Properties and Change: Additional Concepts Topic 3 Separating Mixtures • When precisely controlled, distillation can separate substances having boiling points that differ by only a few degrees. Matter—Properties and Change: Additional Concepts Topic 3 Separating Mixtures • Crystallization is a separation technique that results in the formation of pure solid particles of a substance from a solution containing the dissolved substance. Matter—Properties and Change: Additional Concepts Topic 3 Separating Mixtures • Chromatography is a technique that separates the components of a mixture (called the mobile phase) on the basis of the tendency of each to travel or be drawn across the surface of another material (called the stationary phase). • The separation occurs because the various components of the ink spread through the paper ant different rates. Matter—Properties and Change: Additional Concepts Topic 3 Elements • All matter can be broken down into a relatively small number of basic building blocks called elements. • An element is a pure substance that cannot be separated into simpler substances by physical or chemical means. Matter—Properties and Change: Additional Concepts Topic 3 Elements • Each element has a unique chemical name and symbol. • The chemical symbol consists of one, two, or three letters; the first letter is always capitalized and the remaining letter(s) are always lowercase. Matter—Properties and Change: Additional Concepts Topic 3 Elements • The names and symbols of the elements are universally accepted by scientists in order to make the communication of chemical information possible. Matter—Properties and Change: Additional Concepts Topic 3 Elements • As many new elements were being discovered in the early nineteenth century, chemists began to see patterns of similarities in the chemical and physical properties of particular sets of elements. • Several schemes for organizing the elements on the basis of these similarities were proposed, with varying degrees of success. Matter—Properties and Change: Additional Concepts Topic 3 Elements • In 1869, the Russian chemist Dmitri Mendeleev made a significant contribution to the effort. Mendeleev devised the chart which organized all of the elements that were known at the time into rows and columns based on their similarities and their masses. Matter—Properties and Change: Additional Concepts Topic 3 Elements • Mendeleev’s organizational table was the first version of what has been further developed into the periodic table of elements. • The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups or families. • Elements in the same group have similar chemical and physical properties. Matter—Properties and Change: Additional Concepts Topic 3 Elements • Elements in the same group have similar chemical and physical properties. • The table is called “periodic” because the pattern of similar properties repeats as you move from period to period. Matter—Properties and Change: Additional Concepts Topic 3 Periodic Table Matter—Properties and Change: Additional Concepts Topic 3 Compounds • You know that matter is classified as pure substances and mixtures. • You also know that elements are pure substances that cannot be separated into simpler substances. • There is yet another classification of pure substances—compounds. Matter—Properties and Change: Additional Concepts Topic 3 Compounds • A compound is a combination of two or more different elements that are combined chemically. • Most of the substances that you are familiar with and, in fact, much of the matter of the universe are compounds. • Water, table salt, table sugar, and aspirin are examples of common compounds. Matter—Properties and Change: Additional Concepts Topic 3 Compounds • Today, there are approximately 10 million known compounds, and new compounds continue to be developed and discovered at the rate of about 100 000 per year. Matter—Properties and Change: Additional Concepts Topic 3 Compounds • There appears to be no limit to the number of compounds that can be made or that will be discovered. • Considering this virtually limitless potential, several organizations have assumed the task of collecting data and indexing the known chemical compounds. Matter—Properties and Change: Additional Concepts Topic 3 Compounds • These organizations maintain huge databases that allow researchers to access information on existing compounds. • The databases and retrieval tools enable scientists to build the body of chemical knowledge in an efficient manner. Matter—Properties and Change: Additional Concepts Topic 3 Categories of Matter Matter—Properties and Change: Additional Concepts Topic 3 Compounds • The chemical symbols of the periodic table make it easy to write the formulas for chemical compounds. • For example, table salt, or sodium chloride, is composed of one part sodium (Na) and one part chlorine (Cl), and its chemical formula is NaCl. • Water is composed of two parts hydrogen (H) to one part oxygen (O), and its formula is H2O. Matter—Properties and Change: Additional Concepts Topic 3 Compounds • Unlike elements, compounds can be broken down into simpler substances by chemical means. • In general, compounds that naturally occur are more stable than the individual component elements. • To separate a compound into its elements often requires external energy such as heat or electricity. Matter—Properties and Change: Additional Concepts Topic 3 Compounds • The properties of a compound are different from those of its component elements. The example of water illustrates this fact. • Water is a stable compound that is liquid at room temperature. Matter—Properties and Change: Additional Concepts Topic 3 Compounds • When water is broken down into its components, it is obvious that hydrogen and oxygen are dramatically different than the liquid they form when combined. • Oxygen and hydrogen are tasteless, odorless gases that vigorously undergo chemical reactions with many elements. Matter—Properties and Change: Additional Concepts Topic 3 Law of Definite Proportions • An important characteristic of compounds is that the elements comprising them combine in definite proportions by mass. • This observation is so fundamental that it is summarized as the law of definite proportions. • This law states that, regardless of the amount, a compound is always composed of the same elements in the same proportion by mass. Matter—Properties and Change: Additional Concepts Topic 3 Law of Definite Proportions • The mass of the compound is equal to the sum of the masses of the elements that make up the compound. • The ratio of the mass of each element to the total mass of the compound is a percentage called the percent by mass. Matter—Properties and Change: Additional Concepts Topic 3 Law of Multiple Proportions • The law of multiple proportions states that when different compounds are formed by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in a ratio of small whole numbers. • Ratios compare the relative amounts of any items or substances. Matter—Properties and Change: Additional Concepts Topic 3 Law of Multiple Proportions • The comparison can be expressed using numbers separated by a colon or as a fraction. • With regard to the law of multiple proportions, ratios express the relationship of elements in a compound. Matter—Properties and Change: Additional Concepts Topic 3 Law of Multiple Proportions • The two distinct compounds water (H2O) and hydrogen peroxide (H2O2) illustrate the law of multiple proportions. • Each compound contains the same elements (hydrogen and oxygen). • Water is composed of two parts hydrogen (the element that is present in different amounts in both compounds). Matter—Properties and Change: Additional Concepts Topic 3 Law of Multiple Proportions • Hydrogen peroxide is composed of two parts hydrogen and two parts oxygen. • Hydrogen peroxide differs from water in that it has twice as much oxygen. • When we compare the mass of oxygen in hydrogen peroxide to the mass of oxygen in water, we get the ratio 2:1. Matter—Properties and Change: Additional Concepts Topic 3 Law of Multiple Proportions • Considering that there is a finite number of elements that exist today and an exponentially greater number of compounds that are composed of these elements under various conditions, it becomes clear how important the law of multiple proportions is in chemistry. Additional Assessment Questions Topic 3 Question 1 Identify each of the following as an example of a homogeneous mixture or a heterogeneous mixture. Additional Assessment Questions Topic 3 A. 70% isopropyl rubbing alcohol B. a pile of rusty iron filings C. concrete D. saltwater E. gasoline F. bread Additional Assessment Questions Topic 3 Answers A. 70% isopropyl rubbing alcohol homogeneous B. a pile of rusty iron filings heterogeneous C. concrete heterogeneous D. saltwater homogeneous E. gasoline homogeneous F. bread heterogeneous Additional Assessment Questions Topic 3 Question 2 Identify each of the following as an example of an element or a compound. A. sucrose (table sugar) B. the helium in a balloon C. baking soda D. a diamond Additional Assessment Questions Topic 3 Answers A. sucrose (table sugar) compound B. the helium in a balloon element C. baking soda compound D. a diamond element Additional Assessment Questions Topic 3 Question 3 A 134.50-g sample of aspirin is made up of 6.03 g of hydrogen, 80.70 g of carbon, and 47.77 g of oxygen. What is the percent by mass of each element in aspirin? Additional Assessment Questions Topic 3 Answer 4.48% hydrogen, 60% carbon, 35.52% oxygen Additional Assessment Questions Topic 3 Question 4 A 2.89-g sample of sulfur reacts with 5.72 g of copper to form a black compound. What is the percentage composition of the compound? Additional Assessment Questions Topic 3 Answer 33.6% sulfur, 66.4% copper Help To advance to the next item or next page click on any of the following keys: mouse, space bar, enter, down or forward arrow. Click on this icon to return to the table of contents Click on this icon to return to the previous slide Click on this icon to move to the next slide Click on this icon to open the resources file. Click on this icon to go to the end of the presentation. End of Topic Summary File