Module 6 - The Electron and The Nucleus I.
advertisement
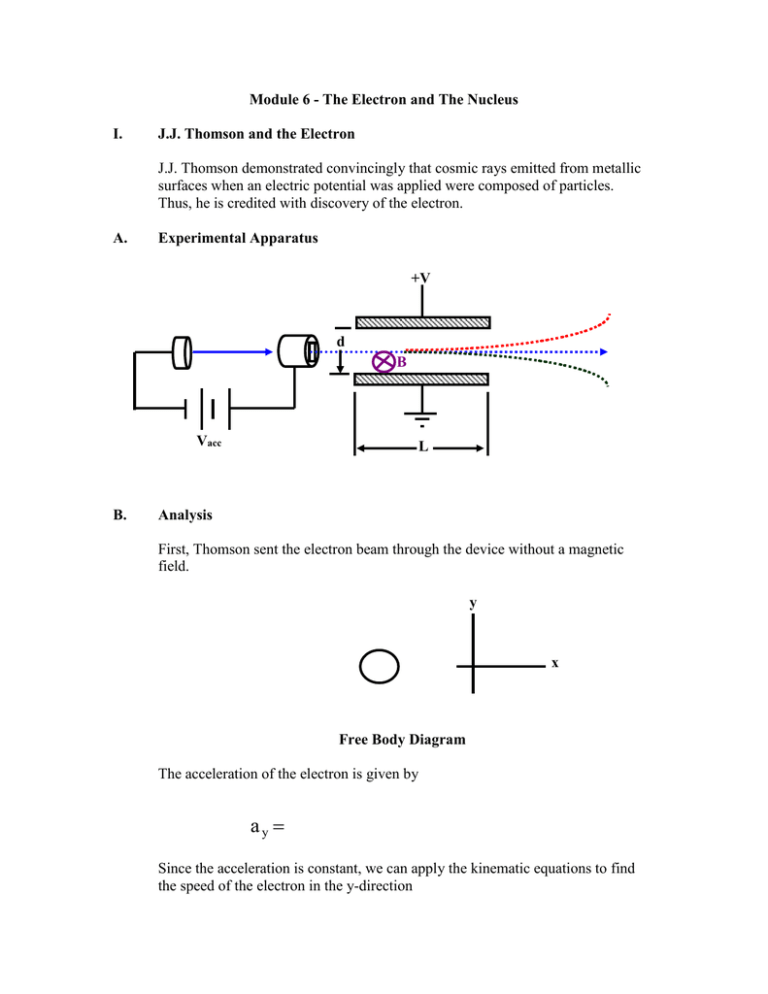
Module 6 - The Electron and The Nucleus I. J.J. Thomson and the Electron J.J. Thomson demonstrated convincingly that cosmic rays emitted from metallic surfaces when an electric potential was applied were composed of particles. Thus, he is credited with discovery of the electron. A. Experimental Apparatus +V d B Vacc B. L Analysis First, Thomson sent the electron beam through the device without a magnetic field. y x Free Body Diagram The acceleration of the electron is given by ay Since the acceleration is constant, we can apply the kinematic equations to find the speed of the electron in the y-direction vy The time that the electron is between the plates can also be determined using the kinematic equations: L= t Using our results, we have a relationship for the charge to mass ratio of an electron: q m Since the deflection of the electron in the y-direction is negligible within the distance spanned by the plates, we have that tan θ Thus, we have a relationship for finding the charge to mass ratio of the electron if we can determine the initial speed of the electron: q v x 2 d tan θ m VL To determine the initial speed of the electron, Thomson used a Wein filter from PHYS2424. With the magnetic field set to cancel the force of the electric field, we have that vx Thus, our final relationship for the charge to mass ratio of the electron is q m V Bd 2 d tan θ VL V tan θ B2 d L For the small angles of deflection, tan θ θ so we have the equation of a straight line with slope q/m as shown below: q B2 d L V m θ (See pg. 12 of Rohlf) II. Thomson Model of the Atom A. Experimental Facts: 1) _____________________________ exist in atoms and are ______________________________ charged. 2) ________________________ is _______________________ so it must also have an ______________________ amount of _________________________ charge. B. Model (Plumb Pudding) III. Alpha Scattering Experiments A. Setup Collimator Radioactive Source Gold Foil B. C. Experimental Facts 1) Most alphas only suffer small angle deflections as expected. 2) A few alphas suffer large angle deflections (incredible result)! Analysis The force between the electric field of the gold atom of charge Z2 and the incoming alpha particle of charge Z1 = 4 is found by F We now use Gauss's Law (PHYS2424) to determine the electric field due to the positive uniformly charged sphere of Thomson's model: R Case I : r < R Case II: r > R The maximum force would occur when the particle is located at the distance r = R as is given by F To determine the impulse, we need to determine the time that the force interacts. We will assume that the force stays at its maximum value for the time required for the alpha to travel across the diameter of the gold atom. This is an approximation but it should over-estimate the alpha deflection and not under-estimate it! 2R Δt Δ p FΔ t We can now determine the angle of deflection by looking at the change in linear momentum diagram p p tan θ Δp p tan θ Using the radius of the atom (1x10-10 m) for the size of the positive charge, the angle of deflection of a 6.0 MeV alpha particle incident upon the gold foil is tan θ 1.44 eV nm 279 3.79 x10 4 θ 0.1nm 6 x106 eV Our results so far consider only a single collision between the alpha particle and a single gold atom in the foil. We can consider the effect of multiple collisions upon the alpha particle. On average, the alpha particle's deflections will cancel out. This is "drunken walk" problem and is described by a Gaussian distribution. The standard deviation is given by θ std Nθ where N is the number of scattering centers. For a 1.0 m gold foil, the number of scattering centers can be approximately determined by 6 1 x 10 m 1x104 N 1x1010 m Thus, we have θ std 1x104 3.79 x10 4 3.79 x102 Summary: Rutherford realized that if the positive charge was concentrated into a smaller radius that the scattering angle is increased. By placing the positive charge in a smaller radius of 1 fm and using classical mechanics, Rutherford developed the following scattering cross section (probability) relationship dσ Z1 Z 2 dΩ E 2 1 sin 4 θ 2 which was verified by careful experiments of his students Geiger and Marsden. IV. Rutherford's (Planetary) Model of the Atom Small Positive Charged Nucleus Electron A. The model successfully explains alpha scattering as well as Thomson's results. B. However, the atom is STABLE while this model is UNSTABLE. A force exists between the electron and the nucleus so the electron must be _________________________________. Thus, the electron must be ______________________________ according to classical E&M. Thus, the electron must lose _____________________ ____________________ and therefore it will ___________________ ______________________. The electron will __________________ into the atom. Classical E&M Picture We would expect to see a continuous spectrum as the electron spiraled into the nucleus in a few fractions of a second.





