Exam 19 , CH8 9 CH 9 12 , CH10 21
advertisement
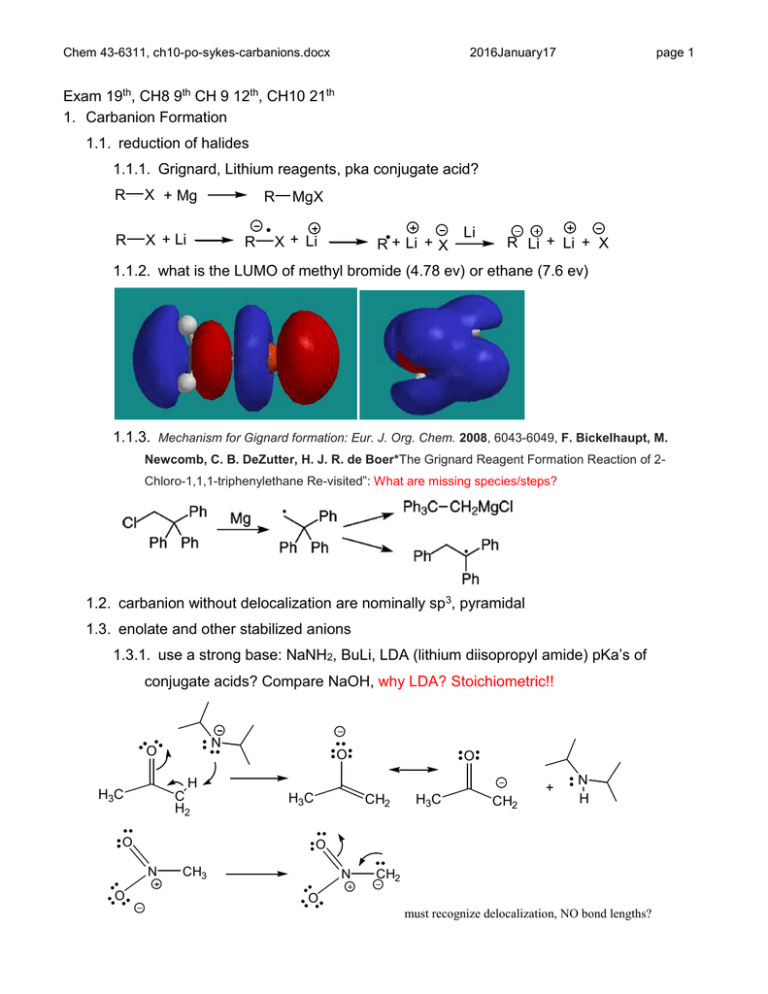
Chem 43-6311, ch10-po-sykes-carbanions.docx 2016January17 page 1 Exam 19th, CH8 9th CH 9 12th, CH10 21th 1. Carbanion Formation 1.1. reduction of halides 1.1.1. Grignard, Lithium reagents, pka conjugate acid? R X + Mg R X + Li R R MgX X + Li R + Li + X Li R Li + Li + X 1.1.2. what is the LUMO of methyl bromide (4.78 ev) or ethane (7.6 ev) 1.1.3. Mechanism for Gignard formation: Eur. J. Org. Chem. 2008, 6043-6049, F. Bickelhaupt, M. Newcomb, C. B. DeZutter, H. J. R. de Boer*The Grignard Reagent Formation Reaction of 2Chloro-1,1,1-triphenylethane Re-visited”: What are missing species/steps? 1.2. carbanion without delocalization are nominally sp3, pyramidal 1.3. enolate and other stabilized anions 1.3.1. use a strong base: NaNH2, BuLi, LDA (lithium diisopropyl amide) pKa’s of conjugate acids? Compare NaOH, why LDA? Stoichiometric!! N O H C H2 H3C O H3C O CH2 H3C + CH2 N H O N O O CH3 N CH2 O must recognize delocalization, NO bond lengths? Chem 43-6311, ch10-po-sykes-carbanions.docx 2016January17 page 2 1.4. geometry restrictions on delocalized anions 1.4.1. sp2 orbitals on carbon, p orbital co planar with orbital 1.4.2. estimate pKa of 1,3-cyclohexandione? 1.4.3. double bond and 4 attached atoms must be coplanar 1.4.4. estimate pKa of 1,3-bicyclo[2.2.2]octandione (What is most acidic CH?) C C C C O C C O O C O O H C C O O O 1.5. enols form reversibly in presence of acid or base catalyst, pka HOAc O O H O OAc C H O H C H HOAc H H H OAc H H HOAc O O H OAc H H H H 1.5.1. enol is isomer of ketone - tautomers – readily interconvertible isomers 1.5.2. tautomerization is normally two step intermolecular process 1.5.3. enol and carbanion in general can be used to label ketone with deuterium 1.5.4. -dicarbonyls have significant enol content fix dash 1.5.5. hydrogen bonding and additional delocalization 1.5.6. solvent is important, 92% enol in hexane, 15% in water, why? Chem 43-6311, ch10-po-sykes-carbanions.docx 2016January17 H O O page 3 O O 1.6. don’t forget reactant state 1.6.1. anti conformation has favorable dipole-dipole interaction 1.6.2. lowers energy relative to pentadione O O O O H O O O H O ~100 % 6x10-3 % 1.7. proton loss is slow even for stronger carbon acids 2. Carboxylation 2.1. organometallic with electropositive metal (Na, Li, Mg) form strong base. HCl CO2 Mg MgBr Br CO2H CO2MgBr 3. Elimination reactions (are possible) 3.1. stabilized anion intermediate, pka? O O O Cl Cl H B O O 4. decarboxylation can form carbanion intermediate, pKa? O Chem 43-6311, ch10-po-sykes-carbanions.docx H3C H H2 C O 2016January17 H2C CO2 NO2 NO2 ClH2C Cl2 O page 4 NO2 NO2 5. alkylation of carbanions 5.1. must avoid elimination as in 3.1, since many carbanions are strong bases 5.2. weak base, pka? O O O O CH3CH2Br H3CO OCH 3 H3CO OCH 3 H H 5.3. use electrophiles that can’t or don’t easily eliminate and base with low steric hindrance 5.3.1. allyl chloride does not have acidic hydrogen to Cl (avoids allene formation) 5.3.2. acetylide is not bulky, but is a very strong base, pka? CH Cl + C CH C 6. Reimer-Tiemann 6.1. Phenol is an enol of 2,4-cyclohexandienone 6.2. enolate is good nucleophile, full mechanism and delocalized electrons as an exercise 6.3. dichlorocarbene: a good electrophile, empty p orbital Chem 43-6311, ch10-po-sykes-carbanions.docx 2016January17 6.4. some para product forms 6.5. Kolbe reaction yields nearly exclusive ortho with sodium counter ion Na O O OH O H C CO2 CO2 O Na Na 6.5.1. para substitution is more important for potassium, why? 7. Darzens reaction 8. Favorski reaction 9. Base-Catalyzed Halogenation of Ketones (figure) O O C H H base + Br 2 H C H Br + HBr H 9.1. rate = kobs[ketone][base] 9.1.1. independent of [Br2], what does this mean? page 5 Chem 43-6311, ch10-po-sykes-carbanions.docx O 2016January17 O C H H page 6 O base + Br Br fast Br C H C H H H Br H 9.2. isotopic labeling 9.2.1. typically rate of exchange with deuterium is the same as racemization 9.2.1.1. then deuteration occurs equally from both sides 9.2.1.2. anion symmetrization must be faster than deuteration O O O C Me H Et fast D2O/ODC slow Et C Et Me Me D 9.3. A deuterium isotope effect is observed 9.4. indicates deprotonation is RDS O O O C Me D Et fast H2O/OHslow C Et Me C Me H Et 9.5. back to halogenation 9.5.1. replacement of subsequent hydrogens by bromine is faster 9.5.2. electron withdrawing Br stabilizes anion 9.6. methyl ketones form bromoform (haloform reaction) 9.6.1. Why doesn’t OH leave instead of CBr3? Experimental test for OH leaving? Chem 43-6311, ch10-po-sykes-carbanions.docx 2016January17 10. Acid catalyzed halogenation, equilibrium at beginning is two steps (see above) 10.1. enol is formed, reacts with halogen 10.2. enol of product less reactive than reactant, bromo enol is poor donor, why? 10.3. Stop at mono-halogenation 10.4. rate is same for iodination, what is RDS? 10.5. what removes proton from cation intermediate? What is the solvent? page 7