1
advertisement

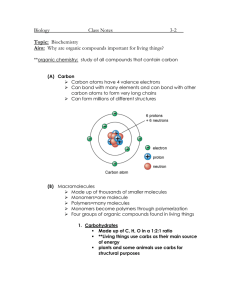
1 Water Properties 2 Carbon Compounds 3 Functional Groups 4 Bonding of Carbon 5 Water & Nature 6 Polymers 7 H2O Properties Carbon Functional compounds Groups Bonding of Carbon $100 $100 $100 $100 $200 $200 $200 $200 $300 $300 $300 Water in Nature Polymers $100 $100 $200 $200 $300 $300 $300 $400 $400 $400 $400 $400 $400 $500 $500 $500 $500 $500 $500 8 Type of bond between hydrogens & oxygen 9 What is covalent? 10 Polar charge on each end of a water molecule 11 What is positive hydrogen & negative oxygen? 12 Overall charge on a water molecule 13 What is zero or no charge? 14 Bonding that causes water to cling to itself 15 What is hydrogen bonding or cohesion? 16 Attraction of water that produces surface tension 17 What is cohesion? 18 Compounds with covalently bonded carbon atoms 19 What is an organic compound? 20 Number of covalent bonds carbon can form 21 What is four? 22 Number of electrons shared in a triple bond 23 What is six? 24 Glucose in cells has this carbon shape 25 What is a ring structure? 26 Carbon shape resulting from the attachment of side groups to a chain 27 What is a branched chain? 28 Effect these groups have on organic compounds 29 What is change the properties? 30 OH group gives this property to organic compounds 31 What is an alcohol or polar? 32 -COOH group 33 What is a carboxyl group? 34 35 -NH2 group is found on this type of macromolecule 36 What are proteins? 37 -PO4 group is found on this macromolecule 38 What are nucleic acids? 39 Small subunits bonded together to make large carbon compounds 40 What are monomers? 41 Simplest type of bond between two carbon atoms 42 What is a single covalent bond? 43 Repeating linked carbon units or monomers make this 44 What is a polymer? 45 Large polymers are called this 46 What are macromolecules? 47 Process that links monomers by removing molecules of water 48 What is condensation or dehydration synthesis? 49 Allows some insects to walk across the top of water 50 What is surface tension? 51 Adhesion and cohesion produce this property in plants 52 What is capillarity? 53 Responsible for the upward movement of water in plants 54 What is capillarity? 55 Must occur for water to change from water into oC ice at 0 56 Lost or gain of a lot of energy 57 Extra energy absorbed by water to change its state breaks these 58 What are hydrogen bonds? 59 Meaning of the prefix poly-? 60 What is many? 61 Process that breaks down polymers 62 What is hydrolysis? 63 Molecule removed to break polymers into monomers 64 What is water? 65 Amino acids link to make this polymer 66 What are polypeptides or proteins? 67 Backbone element in organic polymers 68 What is carbon? 69 70 71 Energy for Cells 72 Carbohydrates 73 Proteins 74 Enzymes 75 Lipids 76 Nucleic Acids 77 Energy for Life Carbohydrates Proteins Enzymes $200 $200 $200 $200 $400 $400 $400 $400 $600 $600 $600 Lipids Nucleic Acids $200 $200 $400 $400 $600 $600 $600 $800 $800 $800 $800 $800 $800 $1000 $1000 $1000 $1000 $1000 $1000 78 Type of carbon to carbon covalent bond storing the most energy 79 What is a quadruple (4) bond? 80 Macromolecules that can store the most energy 81 What are lipids or fats? 82 Original source of energy stored in organic compounds 83 What is sunlight? 84 Energy molecule stored in cells 85 What is ATP? 86 87 Functional group found on ATP 88 What is the phosphate group? 89 Monomer for carbohydrates 90 What are monosaccarhides? 91 Three simple sugars 92 What are glucose, fructose, & galactose? 93 General formula for any monosaccharide 94 What is C6H12O6 or (CH2O)n? 95 Sucrose is this type of carbohydrate 96 What is a disaccharide? 97 Two common polysaccharides found in plants 98 What are cellulose & starch? 99 Monomers of proteins 100 What are amino acids? 101 Bond that forms between amino acids 102 What is a peptide bond? 103 Two functional groups found on amino acids 104 What are the amino (-NH2) & carboxyl (-COOH) groups? 105 Temperate and pH can have this effect on a protein 106 What is change shape or denture? 107 Main difference among the twenty amino acids 108 What are the side or R groups? 109 Enzymes are this type of macromolecule 110 What are proteins? 111 Reactant catalyzed by an enzyme 112 What is a substrate? 113 Effect on the enzyme whenever the reactant joins it 114 What is change shape? 115 Effect on an enzyme of changing pH or temperature 116 What is change the enzyme’s shape? 117 Enzyme lipase digests these 118 What are lipids? 119 Can store lots of energy because of a large number of these 120 What are carbonhydrogen bonds? 121 Hydrophilic end of a fatty acid 122 What is the carboxyl end? 123 124 Alcohol forming the backbone of fats 125 What is glycerol? 126 Makes a fatty acid saturated 127 What is all single bonds between carbons in chain? 128 Two fatty acids joined with a molecule of glycerol in cell membranes 129 What is a phospholipid? 130 Five elements in nucleic acids 131 What is CHONP? 132 Pentose sugar on DNA 133 What is deoxyribose? 134 Copies DNA so proteins can be made 135 What is RNA? 136 Monomers of nucleic acids 137 What are nucleotides? 138 Along with a sugar & phosphate, the rd 3 part of a nucleotide 139 What is a nitrogencontaining base? 140 Final Jeopardy 141 Macromolecules 142 Cholesterol is this type of macromolecule 143 What is a steroid? 144