One-Dimensional (1D) NMR Experiments 1D NMR – - General summary
advertisement
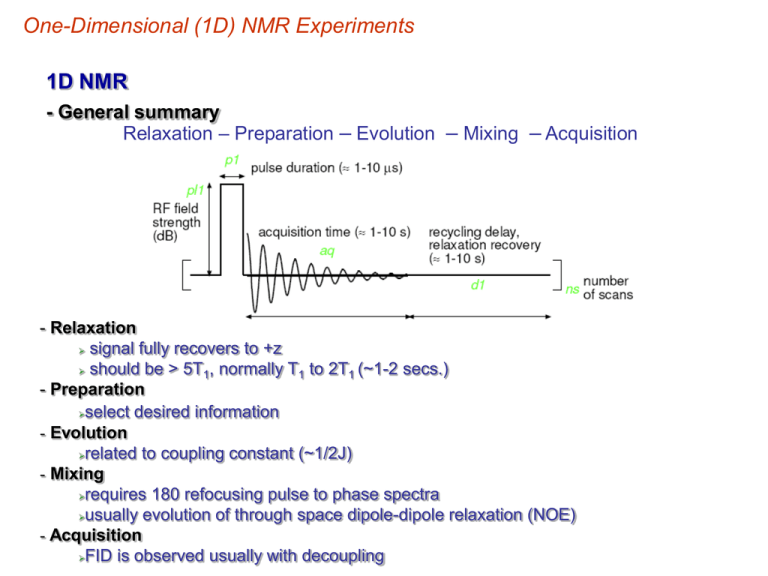
One-Dimensional (1D) NMR Experiments 1D NMR - General summary Relaxation – Preparation – Evolution – Mixing – Acquisition - Relaxation signal fully recovers to +z should be > 5T1, normally T1 to 2T1 (~1-2 secs.) - Preparation select desired information - Evolution related to coupling constant (~1/2J) - Mixing requires 180 refocusing pulse to phase spectra usually evolution of through space dipole-dipole relaxation (NOE) - Acquisition FID is observed usually with decoupling One-Dimensional (1D) NMR Experiments Difference Spectroscopy -Determine which signals change between different experiments vary decoupling frequency change sample composition (protein-ligand titration) change delay times (NOE, coupling) -Subtract the two spectra don’t get perfect cancellation Instrument instability Bloch-Siegert shift Nuclear Overhauser effects Small change in frequency Incomplete cancellation One-Dimensional (1D) NMR Experiments Decoupling Difference Spectroscopy -One spectra collected with decoupling off resonance decoupler set at a frequency far off from any peaks in the spectra -Second spectra collected with selected decoupling of one peak in the spectra -Helps deconvolute complex coupling patterns repeat for each coupled resonance in the spectra - coupled spectra give positive signals - decoupled spectra give negative signals 1H Difference spectrum (b-a) 1H spectrum with Decoupler set on 31P signal of PPh3 1H Reference spectrum signals coupled to 31P One-Dimensional (1D) NMR Experiments Selective Population Transfer -Minimize Bloch-Siegert shift use weak, selective decoupling pulse equalizes population of two spin states effects population of coupled spin states -Changes observed from difference spectra A spins Normal 1:1 A-X doublet 2dN-dN dN-0 0.5:1.5 A-X doublet after selective decoupling 1.5dN-dN 1.5dN-0 One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) -Dipole-dipole relaxation -through space correlation (<5Å) stereochemistry and conformation of molecules NOE 4.1Å 2.9Å -Irradiate one nucleus intensity of nuclei which are close in space change magnitude change depends on nuclei type depends on distance between nuclei Relaxation through interaction of spin-states One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Mechanism for Relaxation • Each nuclei creates a magnetic field that effects other nuclei Dipole-dipole coupling is described by a unit vector that connects the dipoles Field at k created by j • head to tail alignment is lowest energy But structures can constrain relative alignment Magnetic spins are like bar magnets Magnitude of dipole-dipole interaction may come from numerous interactions One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) a) b) Important: effect is time-averaged Gives rise to dipolar relaxation (T1 and T2) and especially to crossrelaxation Mechanism by which spins return to equilibrium state (aligned with external magnetic field +z) Will discuss in detail later in the course Perturb 1H spin population affects 13C spin population NOE effect One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE, h) – the change in intensity of an NMR resonance when the transition of another are perturbed, usually by saturation. hi = (I-Io)/Io where Io is thermal equilibrium intensity Saturation – elimination of a population difference between transitions (irradiating one transition with a weak RF field) irradiate bb ab N N-d X A ba X aa N+d N A Populations and energy levels of a homonuclear AX system (large chemical shift difference) Observed signals only occur from single-quantum transitions One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Saturated (equal population) ab N-½d saturate bb N-½d I S ba I aa N+½d N+½d S Saturated (equal population) Observed signals only occur from single-quantum transitions Populations and energy levels immediately following saturation of the S transitions bb ab N-½d W1A N-½d W1X W2 W0 aa W1X N+½d ba W1A Relaxation back to equilibrium can occur through: Zero-quantum transitions (W0) Single quantum transitions (W1) Double quantum transitions (W2) N+½d The observed NOE will depend on the “rate” of these relaxation pathways One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) bb ab N-½d W1A N-½d X W1 W2 W0 aa Solomon Equation: W1X N+½d ba W1A N+½d W2 W0 X hi A 2W1A W2 W0 Steady-state NOE enhancement at spin A is a function of all the relaxation pathways If only W1, no NOE effect at HA If W0 is dominant, decrease in intensity at HA negative NOE If W2 is dominate, increase in intensity at HA positive NOE For homonuclear (X=A), maximum enhancement is ~ 50% For heteronuclear (X=A), maximum enhancement is ~50%(X/A) One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Intensity of NOE “builds-up” as a function of time (tm – mixing time) NOE build-up rate is dependent on correlation time (tc) and frequency – correlation time: time it takes a molecule to rotate one radian (360o/2p) – ~10-11 secs. for small molecules –~10-9 secs. MW:1000 to 3000 –>10-9 secs. MW > 5000 One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Correlation Time – Debye theory of electric dispersion: 4pa 3 h tc 3k T N – viscosity T – temperature a – radius of molecule k – Boltzman constant Varying temperature, viscosity or mass of sample will change tc One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Mechanism for Relaxation • Dipolar coupling between nuclei and solvent (T1) interaction between nuclear magnetic dipoles depends on correlation time – oscillating magnetic field due to Brownian motion – depends on orientation of the whole molecule in solution, rapid motion averages the dipolar interaction –Brownian motion in crystals, positions are fixed for single molecule, but vary between molecules leading range of frequencies and broad lines. Tumbling of Molecule Creates local Oscillating Magnetic field One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Mechanism for Relaxation • Solvent creates an ensemble of fluctuating magnetic fields causes random precession of nuclei dephasing of spins possibility of energy transfer matching frequency 2t c K (v ) 1 4p 2v 2t c2 Field Intensity at any frequency tc represents the maximum frequency – 10-11s = 1011 rad s-1 = 15920 MHz All lower frequencies are observed One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Mechanism for Relaxation Extreme narrowing limit (flat region) 4p 2v 2 tc ~ 10ns (macromolecule) tc ~ 10ps (small molecule) 1/tc Intensity of fluctuations in magnetic field Proportional to tc (note: different scales) 1 t c2 Relaxation or energy transfers only occurs if some frequencies of motion match the frequency of the energy transition. The available frequencies for a molecule undergoing Brownian tumbling depends on tc. The total “power” available for relaxation is the total area under the spectral density function. One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Mechanism for Relaxation • Spectral density is constant for w << 1/tc tc decreases, wo also decreases and T1 increases at 1/tc ≈ wo there is a point of inflection – W2 falls off first since it is the sum of two transitions relaxation rates via dipolar coupling are: W1A 3t c 3t c r 6 (1 w A2t c2 ) r6 3t c 2t c W0 6 6 2 2 r (w A w X ) t c ) r W2 12t c 12t c r 6 (1 (w A w X ) 2t c2 ) r6 NOE is dependent on the distance (1/r6) separating the two dipole coupled nuclei Important: the effect is time-averaged! Extreme narrowing limit: 1/tc >>wo then wo2tc2 <<1) One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Dependence of NOE on tc • NOE can be positive, zero or negative depending on tc MW Zero NOE positive NOE negative NOE Small molecules Increasing MW Decreasing tc Biomolecules, polymers One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Experimental Aspects of NOE • 50% NOE is theoretically possible • In practice, < 5% NOEs are frequently observed • A number of factors reduces the NOE Any relaxation pathway other than dipole-dipole will reduce NOE – paramagnetic relaxation most common: paramagnetic transition metal ions or O2 degas sample viscous, solvents, MW or presence of solvents lower tc lower hmax NOE builds up by dipole-dipole relaxation – in small molecules, T1DD > 10 secs. To differentiate between NOEs and changes from decoupling, do not decouple during acquisition One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) NOE Difference Spectroscopy • selectively irradiate on resonance intensity will be perturbed for other spatially close nuclei subtract spectra with/without irradiation • Aids in the assignment of the NMR spectra Strong NOE must be H3 Irradiate chemically distinct H7 One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) 13C Spectroscopy • nearly always decoupled to enhance signal to noise lose splitting pattern intensities are not reliable parameter to quantify number of carbons different values of NOE different relaxation times – Quaternary carbons tend to have very long relaxation times and are commonly not observed or severely reduced in intensity • changing when decoupling takes place in pulse sequences can select between, NOE, 1H coupling and full sensitivity enhancement Decoupling with NOE Decoupling with NOE suppression No 1H decoupling One-Dimensional (1D) NMR Experiments Nuclear Overhauser Effect (NOE) Decoupling with NOE suppression NOE while maintaining 1H coupled spectra decouple Decoupling with NOE One-Dimensional (1D) NMR Experiments J Modulation (JMOD) Used to Edit 13C Spectra • changes the “phase” of C and CH2 signals relative to CH and CH3 C and CH2 point up (positive) CH and CH3 point down (negative) • Maximize sensitivity by complete decoupling and NOE, but maintain spin system information. d1 = recycle delay for relaxation d2 = 1/J1H-13C 90o 180o One-Dimensional (1D) NMR Experiments J Modulation (JMOD) Aids in NMR Assignments • Identifies the number of different spin systems 10 presents • Chemical shifts identifies the types of functional groups that are present. 4 3 8 1 6 7 9 2 5 6 8 1 2 4 5 3 7 9,10 One-Dimensional (1D) NMR Experiments J Modulation (JMOD) Remember Coupling constants are in Hz (cycles per second) On resonance (center of coupling pattern) 13C • complete cycle is 360o • each spin moves relative to carrier (center of spin system) during d2 delay 13CH •13C singlet: on resonance doesn’t move during 1/J •13CH doublet each spin distance from carrier is J/2 moves 180o in 1/J •13CH2 triplet: - center peak on-resonance doesn’t move. - outer peaks are J from carrier moves 360o in 1/J •13CH3 quartet: - inner doublet are J/2 from carrier moves 180o in 1/J. Outer - doublet are 3J/2 from carrier moves 540o or an effective 180o in 1/J 13CH 2 13CH 3 180o decouple One-Dimensional (1D) NMR Experiments J Modulation (JMOD) Phase of the Peaks Differ as a result of the Different Spin Systems On resonance (center of coupling pattern) 13C • the 180o pulse and the second 1/J delay allows for refocusing of chemicals shifts that differ from the carrier position rotation is actually dependent on d+J o 180 reverses direction and refocus rotation due to d • 1J13CH ~ 125-170 Hz use average J ~ 145 Hz 13CH of alkynes J ~250 problems with Hz behaves like 13CH2 •Decoupler is turned on during second d2 and acquisition to collapse spins to singlet and gain NOE sensitivity • If d2 set to 1/2J, only observe 13C difficult average J incomplete cancellation and weak 13C signal 13CH 13CH 2 13CH 3 180o decouple One-Dimensional (1D) NMR Experiments INEPT Polarization Transfer • population difference between a and b states is proportional to population difference ~ 4x > 13C 1 13C, 13C S/N would If this difference could be transferred from H to increase by a factor of 4. Lose of NOE effect • polarization transfer > NOE effect 1H One-Dimensional (1D) NMR Experiments INEPT Polarization Transfer • selective 180o on one 1H spin inverts the 1H a and b spin states 13C population differences are now ±DH instead of +DC 1 Repeat by inverting other H spin and subtract spectra in-phase doublet with 4-fold increase in S/N Selective 180o on H1 One-Dimensional (1D) NMR Experiments INEPT Polarization Transfer • Previous described experiment is impractical need to repeat experiment for each unique carbon present in molecule • Can achieve the same effect with the INEPT pulse sequence simultaneous polarization transfer for all carbons present in molecule • Common module of multidimensional NMR experiments 90o 180o 90o 180o 90o d1 = recycle delay for relaxation d2 = 1/4J1H-13C One-Dimensional (1D) NMR Experiments INEPT Separation in peaks indicate triplet (J~145Hz) J -1:1 doublet 13CH INEPT Pascal Triangle 2J -1:0:1 triplet 13CH 2 -1:-1:1:1 quartet 13CH 3 One-Dimensional (1D) NMR Experiments INEPT Decouple INEPT Experiment • results in selective inversion of one spin in the doublet • same result as selective polarization transfer o during first d2 = 1/4J each spin moves 45 o 1H refocusing pulse flips spins (would refocus after another 1/4J delay 180 o 1 180 X pulse exchanges a and b H spins – X attached to a are now attached to b and vice-versa – direction of rotation is reversed o o During second d2, each spin moves another 45 and are aligned 180 to each other 0 90 X pulse generates X FID with polarization transfer phase cycling of receiver can alternatively add and subtract spectra Final 1H 90o will place one spin as +z and the other as –z Effectively, a selective 180o on one spin One-Dimensional (1D) NMR Experiments INEPT Effect of INEPT Pulse Sequence on 1H spins • because spins are 180o to each other, turning on decoupler will cancel spins no signal • insert 180o refocusing pulse separated by d3=1/4J delay 180o refocusing pulse X spin state after standard INEPT (p6) Decoupler turned on X collapse to singlet One-Dimensional (1D) NMR Experiments INEPT Refocused INEPT can Distinguish CH, CH2 and CH3 • selection of d3 as a function of 1/J determines what spins are observed only 13C attached to 1H are observed 0.125/J optimal for all positive signal 13CH observed 0.25/J only 0.375/J CH2 are anti-phase (negative) • common component of multidimensional NMR pulse sequences to select desired correlations • INEPT not commonly used to select spin systems DEPT INEPT is too sensitive to JXH variations CH One-Dimensional (1D) NMR Experiments DEPT Pulse Sequence of Choice to Edit 13C NMR Spectra • not possible to use a simple vector model to explain pulse sequence involves creating multiple-quantum coherence • variable p3 pulse selects desired spin system and phase o 45 pulse: CH, CH2 and CH3 are all positive o 90 pulse: only CH signal observed o 135 pulse CH and CH3 positive with CH2 being negative •Addition and subtraction of DEPT-45, DEPT-90 and DEPT-135 can generate spectra that only contains CH, CH2 or CH3 signals 90o d1 = recycle delay for relaxation d2 = 1/2J1H-13C 180o ao One-Dimensional (1D) NMR Experiments DEPT (DEPT-45 + DEPT-135) – DEPT-90 DEPT-45 - DEPT-135 DEPT-90 Normal Spectra One-Dimensional (1D) NMR Experiments bb A W1 W2 ab W1X W0 W1X DEPT ba W1A aa Wo,W2: multiquantum, forbidden transitions multiple quantum vector does not change during t 13C 90o creates multiple quantum coherence 180o pulse refocus chemical shifts Anti-phase component (amplitude function of sin q) Last 1H pulse Multiquantum component (amplitude function of cos q) One-Dimensional (1D) NMR Experiments PENDANT Pulse Sequence of Choice to Edit 13C NMR Spectra • DEPT does not observe non-protonated 13C atoms • PENDANT same sensitivity as DEPT 13C, 13CH, 13CH and 13CH observes quaternary 2 3 13 quaternary C signals are stronger than in JMOD C/CH2 are opposite phase of CH/CH3 signals • PENDANT with chemical shift information generally sufficient to assign 13C spectrum ambiguities can be removed with the appropriate DEPT experiment • Only requires collecting one spectrum 1 13C spectrum pointless to acquire simple H decoupled replaces JMOD and APT • Again, simple spin vector diagrams are insufficient to describe pulse sequence Creating multiple quantum coherence 90o d1 = recycle delay for relaxation d2 = 1/4J1H-13C d3 = 5/8J1H-13C 180o One-Dimensional (1D) NMR Experiments PENDANT Signals can be Missing from JMOD, INEPT,DEPT or PENDANT • relaxation of peaks occur during delays • worse for broad signals due to exchange or quadrupolar nucleus One-Dimensional (1D) NMR Experiments INADEQUATE Detects Carbon-Carbon Coupling • 13C nuclei only 1.08% abundant weak satellites on either side of strong center peak 13C is 1.17e-2% probability of two bonded atoms both being • Experiment suppresses strong center peak to detect 13C satellites Center peak off-scale 13C C2 satellites C1 C4 C3 C5 Identifying 13C-13C connectivity beneficial for NMR assignment of complex molecules. One-Dimensional (1D) NMR Experiments INADEQUATE Detects Carbon-Carbon Coupling • delay (d2) can be set to select 1J13C-13C or longer coupling 13C-13C •Two-dimensional version (2D) determines 13C-13C connectivity d1 = recycle delay for relaxation d2 = 1/4J13C-13C 1H decoupling on throughout experiment One-Dimensional (1D) NMR Experiments INADEQUATE d2 = 0.08 sec J13C-13C = 3 Hz d2 = 0.0062 sec J13C-13C = 40 Hz 13C spectrum One-Dimensional (1D) NMR Experiments Summary of Information Present in Some 1D Experiments







