Chapter 3: Matter & Energy
advertisement

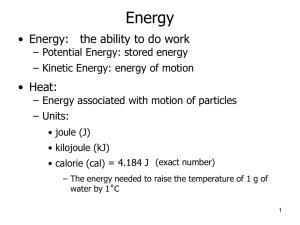
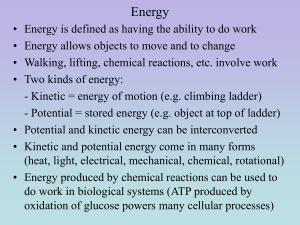
DE CHEMISTRY: King William High School Pure substances (elements & compounds) have definite composition Mixture is two or more different substances physically combined Homogeneous mixtures – uniform throughout (solution) Heterogeneous mixtures – NOT uniform Solid – definite shape and volume Liquid – definite volume; NOT shape Gas – neither definite shape nor volume Physical properties – shape, color, MP, BP, FP Chemical properties – describe a substance changing into something new (metal rusting) Physical change – changes in state (melting, freezing, etc.) Chemical change – changes into a new substance Kinetic v. potential Heat- energy associated with the motion of molecules Units (Joule and calorie) 1 cal = 4.184 J 1Cal = 1 kcal = 1000 cal Convert 6.2 x 102 J to cal and Cal. Carbohydrate = 4kcal/g Fat = 9 kcal/g Protein = 4 kcal/g EX: You eat a meal that has 37 g of carbs, 19 g of fat and 23 g of protein. How many Calories did you intake? Q = cmDT Table 3.11 on page 74 How much heat is released when 7 g of copper is cooled from 20oC to 10oC? Melting and freezing points Heat of fusion – energy needed to melt a substance Heat = mass x heat of fusion How much heat will be absorbed from 26.0 g of ice in a soft drink at 0oC if the heat of fusion = 334 j/g? Heat of vaporization – energy required to turn a liquid to a gas at its BP Heat = mass x heat of vap. Page 81 Opposite of heating curves How much energy is needed to heat 100 g of diethyl ether from 5oC to its boiling point and boil it? The specific heat is 2.33 J/goC. The heat of vaporization is 357 j/g.