Student will learn: 4 periodic trends : atomic radii trend ionization trend
advertisement

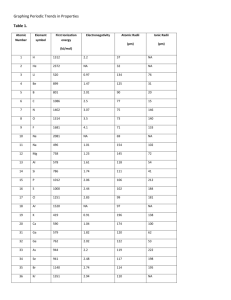
Student will learn: 4 periodic trends : atomic radii trend ionization trend electronegativity trend reactivity trend Periodic Trends Atomic Radius ½ distance between nuclei of identical atoms that are bonded Ionization Energy Energy required to remove one electron Electronegativity Atom’s ability to gain an electron. Reactivity Atomic Radii Period trends Trend to larger radii because proton pull is less on e- of same energy level Group trends Trend to larger atoms because e- are added to NEXT energy level … farther away from nucleus. Which has largest atomic radii? Mg, Cl, Na, P, Which has largest atomic radii? Ca, Be, Ba, Sr? Which has smallest atomic radii? Li, O, C, F ? 88888 Octet rule • Chemical compounds tend to form so that each atom has an octet of electrons in its highest occupied energy level. … • ….atoms will try to get a group of 8 electrons in its outer orbit…. • Either by gaining, losing, sharing electrons Ion = an atom or group of bonded atoms that have a + or – charge. lost an electron = postive……Na+ cation gain an electron =negative…Cl• Do oxidation numbers here anion. Radii of Ions Positive Cations lost e- …. » Lost therefore radii smaller Negative Anion gained e- …. » gained therefore radii larger. Describe the ion radii of the following as increase or decrease. Cl, N, Ca, Cu, S, Na, Ionization energy energy required to remove one electron energy required to make it a cation Period trends Increases across period because more protons pull greater on e- in same energy level Group trends Increases going up group. Electrons are closer to nucleus so protons pull electrons Tighter therefore takes more energy to take an e-. Measured in k/joules 1st Ionization = energy required to remove 1st electron. 2nd ionization = energy required to remove 2nd electron. 3rd ionization = energy required to remove 3rd electron. “numbers will increase” “Arrange the following in increasing ionization energy.” Li, O, C, K, Ne, F K,Li,C,O,F,Ne Be, Ba,Ca,Mg Ba,Ca,Mg,Be Which would least likely form? Sr2+, Al3+, K2+ K2+ Electronegativity ability to gain electrons ability to become an anion Period: increases across period b/c octet rule is being filled Group: increases going up b/c electron closer to pull of protons Which element would gain e- easier? Br, I, Cl, F Which element is most electronegative? C, N, O, Se, S • Reactivity Which would react easier with chlorine? Na or K How about Sodium and Magnesium? If Li and K and Br were mixed in a beaker. What compound would be the largest percentage? 1. In a given group lowest first ionization energy is a. Noble gas b. Halogens c. Alkali metal d. Alkaline earth metals 2. a. b. c. d. Which element has an ion with a radius that is larger than its atomic radius? K Cr Zn Cl 3.Which element in period 3 has the greatest tendancy to gain electrons? a. Ar b. Cl c. Si d. Na 4. a. b. c. d. Arrange the following in increasing order of atomic radii. Al, Si, P Li, Na, K I, Br, Cl N, O, F 5. a. b. c. d. Which particle has the smallest radii? Na K Na+ K+ 6. Which element would be the most reactive with water? Au Mg Fe Na a. b. c. d. Student will learn: 4 periodic trends : atomic radii trend ionization trend electronegativity trend reactivity trend