Macromolecules (Network Covalent) Last part of Topic 4.3
advertisement

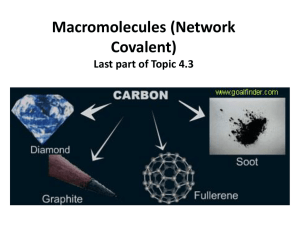
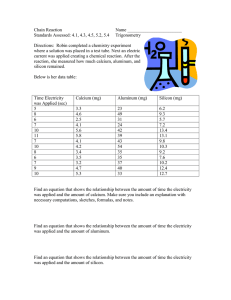
Macromolecules (Network Covalent) Last part of Topic 4.3 • characteristics – some elements from group 14 (carbon and silicon form giant macromolecules • the entire piece is one molecule – insoluble in almost all solvents 2 Carbon allotropes • the same element can be covalently bonded in four of different forms (allotropes) – covalent network solids- atoms are held together by covalent bonds in a 3-D lattice structure that “go on forever” 1. graphite 2. graphene 3. diamond – molecular and have a definite formula 4. fullerenes Graphene- The super material! • strongly bonded carbon in a trigonal planar structure (120°) • one of the thinnest and strongest known materials – only one atom thick, a two dimensional crystal • superb conductor of electricity • can be rolled up to form a carbon nanotube or folded into a sphere (fullerene) Graphite • multiple layers of graphene • between the layers are weak bonds (called London forces) – electricity can easily move between the sheets since it has freely moving charged particles (electrons) – layers can slide past one another therefore a good lubricant – brittle and high melting point Fullerenes • has some delocalized (moving) electrons but cannot conduct electricity since electrons can’t move from one molecule to the next • most famous is the C60 fullerene often referred to as buckeyballs – 60 carbons in 12 pentagons and 20 hexagons • can form a hollow, cage-like structure or tubes Diamond • repeating tetrahedral structure (109.5°) • one of the hardest materials known • very high melting point • no free moving electrons since they are being shared in covalent bonds – cannot conduct electricity see all the tetrahedrals put together Silicon and silicon dioxide • both (Si and SiO2) are very “strong” structures • very high melting point • very high boiling point –silicon • giant (macro) molecules made up of tetrahedrals covalently bonded together • this structure is repeated over and over silicon is in group 14 just like carbon, so notice the same covalent network solid shape – silicon dioxide (sand) • same structure as silicon but each silicon atom is bridged to its neighbors by an oxygen atom • most common crystalline form is quartz • high melting and boiling point • does not conduct electricity