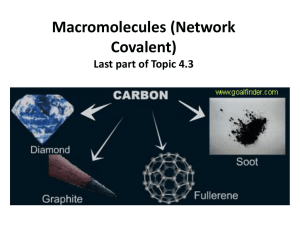
a) Describe and explain the variation of physical and chemical properties of group 14 elements and their compounds The carbon family, (Group 14) in the p-block, contains carbon (C), silicon (Si), germanium (Ge), tin (Sn)and lead (Pb). Each of these elements has only two electrons in its outermost p orbital each. The Group 14 elements tend to adopt oxidation states of +4 and, for the heavier elements, +2 due to the inert pair effect. Members of this group conform well to general periodic trends. Atomic radii increase down the group, and ionization energies decrease. Metallic properties increase down the group. Carbon is a non-metal, silicon and germanium are metalloids, and tin and lead are poor metals (they conduct heat and electricity less effectively than other metals such as copper). Despite their adherence to periodic trends, the properties of the carbon family vary greatly in both physical and chemical properties such as density, melting point and boiling point, electrical conductivity thus for physical properties and for chemical properties includes their reactivities Melting and boiling points of group 14 Melting Points and Boiling Points The melting points and boiling points of group 14 elements decrease as we go down the group, thus due to the change from covalent to metallic bonding. Carbon and silicon have strong covalent bond which results in formation of giant molecular structures which requires a lot of energy to break, hence higher melting points. The trend ultimately shows that as the atoms get bigger and bonds get longer, the bonds get weaker Brittleness Carbon as diamond is very hard, but if hit with a hammer it can easily shatter In other words, there is near no flexibility in the carbon bonds of diamond silicon, germanium, and grey tin (form of tin existing in a covalent structure) are also very brittle. White tin and lead have metallic structures, and thus the atoms can roll over each other without disrupting the metallic bonds, thus they are malleable and ductile, properties of metals Electrical conductivity In diamond form has no electrical conductivity, since the electrons are too tightly bound to move freely graphite is more of an anomaly than an antithesis, due to the unique structure of graphite which can slide over each other hence it has free electrons which can conduct electricity. Silicon, germanium, and grey tin are semiconductors can conduct electricity if enough energy is passed through. When lots of atoms come together to form a large structure, their atomic orbitals merge thus producing a large number of molecular orbitals. These orbitals arrange in bands of increasing energy in terms of reference, one band is known as a valence band - Hold the electrons which make the normal covalent or metallic bonding - And the other the Conductance Band - A higher energy level band - As temperature increases, electrons gain thermal energy, and some electrons may jump from the valence band into the conductance band if the distance is small enough - Once the electrons reach the conductance band, they are delocalized and can freely move to conduct electricity Diamond - The energy gap between the valence band and the conductance band is too high for such a jump to occur Silicon - In silicon, and the same for Germanium and Grey Tin the band gap is small enough for electrons to jump, and thus silicon is a semiconductor White Tin and Lead - They act as normal metallic conductors of electricity as described by the properties of metallic bonds found here The Trend - Thus, the trend of the non-metallic non-conductance behaviour of Carbon to the conductance behaviour of white tin and lead can be clearly seen throughout the Group 4 elements Chemical properties The reactivity of elements decreases down the group. The inert pair effect becomes the reactivity of elements decreases down the group. The inert pair effect becomes increasingly effective down the group. The stability of the +4oxidation state decreases increasingly effective down the group. The stability of the +4 oxidation state decreases. BRIGGS 5thedition while that of the +2 oxidation state increases on descending the group, while that of the +2 oxidation state increases on descending the group .E .g Sn2+ exist as simple ion and is strongly reducing , Sn4+ is covalent ,Pb2+ is ionic ,stable and more common than Pb4+ . Reaction with water C, Si and Ge are unaffected by water, Sn reacts with steam to give SnO, C, Si and Ge are unaffected by water. Sn reacts with steam to give SnO and H and H . Pb is unaffected by water due to the formation of protective oxide layer at the surface. Reaction with acids C, Si and Ge are not affected by dilute acids. Sn and Pb reacts with dilute nitric acid .C, Si and Ge are not affected by dilute acids, Sn and Pb reacts with dilute nitric. Application in chemistry Birk, James 1989.The oxides of group 14, some of them can act as acids when mixed with alkali 4Sn + 10HNO3 4Sn(NO3)2 + NH4NO3 + 3H2O 3Pb + 8HNO3 3Pb(NO3)2 + 2NO +4H2O Reaction of group 14 oxides with acid XO2 + 2NaOH Na2XO3 + H2O Thus for Ge, Sn and Pb for all reactions, hot and concentrated sodium hydroxide is used Reaction with alkalis C is not affected by alkalis. C is not affected by alkalis. Si reacts with alkalis forming silicates. Si reacts with alkalis forming silicates. Sn and Pb also react with alkalis forming stannate, [Sn(OH)6] ,and plumbate , [Pb(OH)6]2-.These reactions show that Sn and Pb are amphoteric. These reactions show that Sn and Pb are amphoteric. Reactions with halogens Graphite but not diamond is affected by F2 at higher temperatures giving (CF)n Si and Ge react with all halogens, forming volatile SiX4 and GeX4. Sn and Pb are less reactive but do react giving SnX4 and PbX2. Sn + 2X2 Pb + Cl2 SnX4 PbCl2 b) Relationships in the periodic table are similarities between pairs of elements in different groups and periods. By reference to the first three elements of the second period9Li,Be and B),their physical properties and relevant balanced chemical equations ,describe the similarities . Li and Mg form only normal oxides whereas Na forms peroxide and metal below Na in addition form super oxides. Li is the only group 1 element which forms a stable nitride, Li3N and Mg as well as other group 2 elements also form nitrides 6Lis+N2g 3Mgs+N2s 2Li3NS Mg3N2s Li and Mg can form stable nitride. They can also form stable oxides thus 4Li + O2 2Mg + O2 2Li2O 2MgO Lithium Carbonate is sparingly insoluble in water and group 2 elements also dissolve Li2CO3 MgCO3 Li2O + CO2 MgO + CO2 Both Li and Mg form covalent organo-metallic compounds. LiMe and MgMe2 are both valuable synthetic reagents .Both Li and Mg chlorides are deliquescent(absorb moisture from surroundings) and soluble in alcohol and pyridine .LiCl ,like (MgCl.6H2O) separate from hydrated crystal LiCl.2H2O.Thus the chemistry of Li has similarities to those of Mg . Reaction of group 2 Clark, Jim (2005) and Inorganic chemistry Shrive, Duward (2006) B2O3 is an acidic oxide, like SiO2 B2O3 + 6NaOH SiO2 + 2NaOH 2Na3BO3+ H2O Na2SiO3 + H2O Boron shows anomalous behaviour in its group because of its small size and non-availability of d orbitals .It resembles silicon ,the second member of the next higher group .Both B and Si are non-metals and exist in allotropic forms .They have high melting points and are semi- conductors .The other members of group 13 are metals . Both boron and silicon form halides which are readily hydrolysed BCl3 + 3H2O B(OH)3 +3HCl Boric acid SiCl4 + 4H2O Si(OH)4 + 4HCl Silic acid Both boron and silicon form several volatile and spontaneously inflammable hydrides called boranes and silanes ,respectively .The hydrides are readily hydrolysed .The lower hydrides can be obtained by the reduction of chlorine with LiAlH4 4BCl3+ 3LiAlH4 SiCl4 + LiAlH4 3AlCl3 + 3LiCl + 2B2H6 AlCl3 + LiCl + SiH4 Be and Al also has some similarities .They all have the tendency to form covalent compounds e .g chlorides of both being covalent are soluble in organic solvents .Both BeCl2 and AlCl3 act as a Lewis acids are used as Friedel-Crafts catalyst .Inorganic chemistry Shrive ,Duward 2006.Both Be and Cl are resistant to the acid action due to formation of protective film of the oxides on their surface The oxides of both beryllium (BeO) and Aluminium(Al2O3) are hard and have high melting solids .They are also amphoteric and dissolve in NaOH solution as well e .g for HCl Be0 + 2HCl BeCl2 + H2O Be0 + 2NaOH Na2BeO2 + H2O Al2O3 + 6HCl 2AlCl3 + 3H2O Al2O3 + 2NaOH 2NaAlO2 + H2O References Briggs 5th edi