Organic Molecules: Lipids and Carbohydrates
advertisement
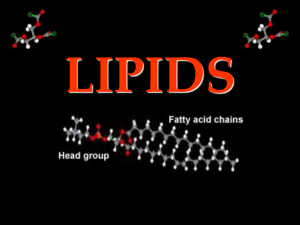
Organic Molecules: Lipids and Carbohydrates Carbon-Based Life Form Carbon is the backbone for all life on Earth. Carbon forms the compounds that make up all cells and organisms. Carbon-Based Life Form Organic molecules make up living things. These molecules are composed of carbon and hydrogen. Sometimes they also will have oxygen, nitrogen, phosphorus & other elements The Uniqueness of Carbon Atoms create molecules by forming covalent bonds – sharing electrons. The number of covalent bonds depends upon the atoms that are interacting. Hydrogen – can only create one bond since it only has one electron to share. The Uniqueness of Carbon Carbon is unique because it readily makes four bonds with other atoms! The Uniqueness of Carbon Be H H2N X X H O OH aware with the state asking a question that looks like this: What does the X represent in this molecule? The Uniqueness of Carbon Carbon atoms are most stable when they have all four bonds filled. Typically, carbon makes single bonds – like that in methane. However, carbon can also form double and triple bonds. Carbon Dioxide Acetylene The Uniqueness of Carbon Carbon’s unique structure permits the formation of four different important macromolecules: 1. Lipids 2. Nucleic Acids 3. Carbohydrates 4. Proteins Lipids: Fats and Oils Lipids include oils, waxes, fats and sterols. Sterols include cholesterol. Lipids: Fats and Oils Fats and oils share the same monomer: fatty acids attached to a single glycerol molecule. Monomers are basic structures of macromolecules! Lipids: Fats and Oils Fatty acids are long chains of carbon atoms connected to each other with double or single bonds. Lipids are mostly composed of carbon and hydrogen with a few oxygen atoms. Glycerol Fatty acids Lipids: Fats and Oils Two forms of fatty acids: saturated (bad) and unsaturated (better). Saturated fats has carbon atoms held together with the maximum number of single bonds and hydrogen atoms. Unsaturated fats has carbon atoms held together with single and double bonds with hydrogen atoms. Lipid Functions Long-term energy storage: In animals, such as us, we store lipids in adipose tissue. Adipose tissue contains special cells for lipid storage. Lipid Functions Cell Membranes: Phospholipids help to form the cell membrane and the organelles membranes in cells. Phospholipids are composed of a polar phosphate group attached to a non-polar glycerol. Cell membranes are also called plasma membranes! Polar Non-Polar Lipid Functions Chemical Messengers: Sterols act as chemical messengers. Cholesterol is the monomer of estrogen, progesterone, and testosterone. Phospholipids The cell membrane (plasma membrane) is made up of phospholipids. Two fatty acid make up the non-polar tails. The phosphate group connects the two fatty acids and makes the polar head. Phospholipids The lipids in cell membranes make them impermeable to water. Water can not freely cross the cell membrane. Water must pass through special protein channels. Phospholipids When we talk about the cell membrane we say that the polar heads are hydrophilic and the non-polar tails are hydrophobic. 1. What is hydrophilic? 2. What is hydrophobic? Phospholipids The plasma membrane is made up with a phospholipid bilayer, or two sheets of phospholipids. Given the structure of a phospholipid, how are these sheets arranged in the plasma membrane? 1. head*tail – head*tail 2. head*tail – tail*head 3. tail*head – tail*head 4. tail*head – head*tail Cell Membrane Exterior of cell ?*? - ?*? Cytoplasm Carbohydrates Carbohydrates consist of carbon, hydrogen and oxygen with a 1:2:1 ratio. Examples: Glucose: C6H12O6 Sucrose: C12H22O11 Carbohydrates Monosaccharide The simplest form of a carbohydrate is glucose. The carbohydrate’s monomer is called a monosaccharide – glucose is a monosaccharide. Monosaccharides single rings. tend to be Single ring Carbohydrates Monosaccharide Table sugar (sucrose) is a disaccharide formed by bonding two monosaccharides in a double ring structure. Single ring Disaccharide Double ring Carbohydrates Many monosaccharides tend to be isomers. Isomers are molecules that have the same chemical formula but different arrangement of atoms. Polysaccharides Monosaccharides can be linked together to form long chains known as polysaccharides. Polysaccharides as polymers. Many are known glucose molecules link together to form starch, glycogen and cellulose. Polysaccharides Starch is a polysaccharide produced by plants to store energy. Starch is found in wheat, rice and potatoes. Polysaccharides Cellulose is a polysaccharide that composes the plant’s cell wall. It strengthens and supports the plant cell. It can only be broken down by very few organisms. Many Functions of Carbohydrates Cellular respiration: all carbohydrates are broken down into glucose to be used in cellular respiration for ATP production. Any time you see energy production in cells … immediately think ATP! Many Functions of Carbohydrates Energy storage: Plants and animals store energy in the form of carbohydrates called glycogen and starch. Structure: plant cell walls are strengthened by cellulose. Carbohydrate Reactions Organisms depend upon chemical reactions to join monomers together and break polymers apart. Dehydration synthesis joins two monomers together. Hydrolysis breaks down polymers into monomers AWAY FROM WATER PROCESS Dehydration Synthesis Dehydration synthesis links two monosaccharides such as glucose and fructose into larger molecules. When this occurs, glucose loses a hydrogen and fructose loses a oxygen and a hydrogen. Along with the new disaccharide being formed, a water molecule is formed. H+ + OH- = H2O Dehydration Synthesis When dehydration synthesis occurs repeatedly longer chains of carbohydrates are formed. Dehydration synthesis also joins fatty acids to the glycerol molecule in lipids. L I P I D S C A R B S Water Breakdown Process Hydrolysis Hydrolysis breaks down polysaccharides into monosaccharides. Water is used to add back the –OH group and H+ atom to the monomers. Hydrolysis is the reverse reaction of dehydration synthesis Carbohydrate Reactions In a cell, 132 glucose monomers are joined to form a straight chain of starch. Remember, for dehydration synthesis to occur, water is formed. How many water molecules are formed in this situation?