Introduction to Organic and Biochemistry (CHE 124) Reading Assignment
advertisement

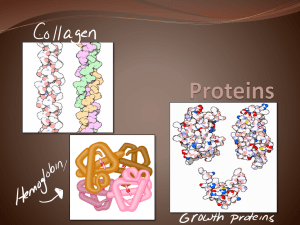
Introduction to Organic and Biochemistry (CHE 124) Reading Assignment General, Organic, and Biological Chemistry: An Integrated Approach 3rd. Ed. Ramond Chapter 12 Peptides, Proteins, and Enzymes Protein • Linear, unbranched polymer of 50 or more amino acids. Amino acids are connected through peptide bonds (amide linkage). • The linear sequence of amino acids folds into a three dimensional structure. Functions of Proteins • Enzymatic catalysis most chemical reactions in the cell are carried out by enzymes ,which are globular proteins. They increase the rate of chemical reactions by reducing the energy of activation. • Transport and storage small molecules are moved throughout the cell by specific transporters. ie hemoglobin transports O2 in the blood. • Mechanical support the high tensile strength of bone and skin is due to the presence of collagen, a fibrous protein. • Coordinate motion muscles are made mostly of proteins. Contraction of muscles relies on the sliding motion of two types of proteins, actin and myosin. Motion of a flagella and movement of chromosomes in mitosis also relies on proteins. Functions of Proteins • Generate and transmission of nerve impulses sending and receiving messages between nerve cells requires receptor proteins that detects the presence of acetylcholine. • Control of growth and differentiation proteins turn the expression of other proteins on and off by binding to specific sequences on DNA. • Immune protection antibodies are highly specific proteins that identify and remove foreign substances from the cell. Definitions • Glucogenic Amino Acids – Carbon skeleton is converted into intermediate(s) that can be used to synthesize glucose. • Ketogenic Amino Acids – Carbon skeleton is converted into intermediate(s) (acetyl CoA or Acetoacetyl CoA) that can form ketone bodies or fatty acids – NOT substrate for glyconeogenesis • Nonessential Amino Acids – enzymes present for de novo synthesis of these amino acids • Essential Amino Acids – (TV FILM HWK) – Organism lacks enzymes to synthesize the amino acids. Amino Acid Backbone • 20 naturally occurring amino acids are incorporated into proteins. • Living systems contain – L amino acids Amino Acids • GLUCOGENIC BOTH NONESSENTIAL – Alanine (A, Ala) – Arginine (R, Arg)* – Asparagine (N,Asn ) – Aspartate (D, Asp) – Cysteine (C, Cys) – Glutamate (E, Glu) – Glutamine (Q, Gln) – Glycine (G, Gly) – Proline (P, Pro) – Serine (S, Ser) NONESSENTIAL – Tyrosine (Y, Tyr) ESSENTIAL ESSENTIAL (Val and His Three Methods) – – – – Histidine (H, His)* Methionine (M, Met) Threonin (T, Thr) Valine (V, Val) Essential: TV FILM HWK (Iley Trpped BOTH Phesants) Lysine – Isoleucine (I, ile) – Phenylalanine (F, Phe) – Tryptophane (W, Trp) KETOGENIC NONESSENTAIL ESSENTAIL KETONES in Leu of --Leucine (L, Leu) -- Lysine (K, Lys) Updated 2014 Cyclic (1) Name Proline Structure M.W. One Three Type R group pKa 75 P Pro Nonpolar ---- • Proline is associated with bends, kinks or tight turns in protein structure. • Often followed by glycine in hairpin turns. Aliphatic (5) Name Structure MW One Glycine Three Type R group pKa 75 G Gly Nonpolar ---- Alanine 89 A Ala Nonpolar ---- Valine 117 V Val Nonpolar ---- Methyl Aliphatic (Cont’) Name MW One Three Leucine 131 L Leu Nonpolar Isoleucine 131 I Ile • Aliphatic- Structure Type R group pKa Nonpolar Nonaromatic hydrocarbon (H and C only). – Nonpolar, hydrophobic • Participate in hydrophobic interactions. • Usually found inside proteins, away from the aqueous solvent, ---- ---- Sulfur Containing (2) Name Cysteine Methionine Structure Sulfhydryl MW One 121 C 149 M Three Type R group pKa Cys Nonpolar 8.3 Met ---- Nonpolar • Cysteine may form disulfide bridges, which stabilize 3’ structure. • Methionine is first amino acid incorporated in growing peptide during translation. Aromatic (3) Name Phenylalanine Structure Phenyl Tryptophane Indole MW One Three Type 165 F Phe Nonpolar ---- 204 W Trp ---- Nonpolar R group pKa Aromatic (cont’) (Tyr is also hydroxyl containing) Name Tyrosine Structure MW One Three Type R group pKa 181 Y Tyr Polar 10.9 Aromatic • • • • May participate in hydrophobic bonding May bind planar ligands via van der Waals stacking Absorb Ultraviolet light (approx. 280 n.m.) Tyrosine may hydrogen bond or donate a proton in catalysis Hydroxyl Containing (3) Name Serine Threonine • • • • Structure Hydroxyl MW One Three Type R group pKa 105 S Ser Polar ---- 119 T Thr Polar ---- Alcohols or hydroxyl-containing side chains Proton donors. Ser is at active site of some enzymes. Attachment of O-linked carbohydrates to proteins Acidic (2) Name Aspartic Acid (Aspartate) Glutamic Acid (Glutamate) Structure MW One Three Type R group pKa 133 D Asp Polar (Acidic) 3.9 147 E Glu Polar (Acidic) 4.3 Carboxylic acid Carboxylic acid • Polar – charged (acidic), hydrophilic. • Found at the Surface of proteins • Often at active site of enzymes to donate / accept a proton. Neutral Amide (2) Name Asparagine Glutamine Structure Amido Amido • Polar – uncharged. • Participates in hydrogen bonding. MW One Three Type R group pKa 132 N Asn Polar ---- 146 Q Gln Polar ---- Basic (3) Name Lysine Arginine Structure MW One Three Type R group pKa 146 K Lys Polar (Basic) 10.8 174 R Arg Polar (Basic) 12.5 155 H His Polar (Basic) 6.0 Amino Guanidinium Imidazole Histidine ● ● Polar – charged (basic), hydrophilic. Located on the surface. May be involved in catalysis or metal binding. Amino acids may exist as stereoisomers The amino acid alpha carbon is chiral (except G) • S (sinister) = left = counterclockwise • R (rectus) = right = clockwise – Most L amino acids have an S absolute configuration The charge of an amino acid changes with pH Amino acid residues are connected by peptide bonds • Peptide bond – linear and planar – not free to rotate • Resonance – partial double bond characteristics (Can you draw a resonance structure?) • Trans – Hydrogen of the substituted amino group is trans to the oxygen of the carbonyl group • exception X-pro The peptide bond exhibit resonance and, therefore, possesses double bond character • Peptide bond is planar with a bond length of 1.32 Å. – Intermediate between C-N (1.49 Å) and C=N (1.27 Å) • Peptide bond is uncharged. Protein has amino (N) terminal and carboxyl (C) terminal end Four Levels of Protein Structure • Primary Structure – linear sequence of amino acids. • N-met-ala-pro-gly-asp-ala-his -C • Secondary Structure α (alpha) helix β (beta) sheets β (beta) turns Ω (omega) loop – hydrogen bonding between the carboxyl oxygen and nitrogen hydrogen of the peptide chain (back bone) • Tertiary Structure – folding of polypeptide chain as a result of interactions between R-groups – A domain is a unit of tertiary structure • • • • • helix turn helix. helix loop helix zinc fingers leucine zipper Quaternary Structure – interaction of different polypeptide chains (subunits) to form a functional protein. Alpha Helix: type of secondary structure Proposed by Linus Pauling and Robert Corey (1951) • Orientation is right handed helix • • • Stabilized by hydrogen bonding between – – carbonyl oxygen NH group of peptide • • every fourth amino acid 3.6 amino acids per turn of helix – • • Right hand = clockwise Left hand = counterclockwise Translation of 1.5 Å and rotation of 100 degrees. R- groups extend outward Helix is disrupted by • proline • large number of charged amino acids – (e.g. Q, E, H, K, R) • amino acids with bulky side chains – (e.g.W) • amino acids with branched R groups – (e.g. V,I) Helix turn Helix: A type of Domain Beta Pleated Sheet: type of secondary structure Proposed by Pauling and Corey (1951) • • • • • Orientation of proteins is flat or pleated, linear “sheet” of proteins. Stabilized by hydrogen bonding between – Carbonyl oxygen – NH group of peptide Adjacent amino acids are separated by 3.5 Å Strands may organize themselves into several orientations – Antiparallel – Parallel – Mixed (both antiparrellel and parallel) Beta bends – contain proline and glycine Tertiary Structure • Tertiary Structure – folding of polypeptide chain as a result of interactions between R-groups Quaternary Structure • Quaternary Structure – interaction of different polypeptide chains (subunits) to form a functional protein. – Myoglobin will be discussed later along with hemoglobin – Hemoglobin is a α2β2 tetramer Denaturing Protein • unfolding and disorganization of a proteins secondary and tertiary structure. – Does not involve hydrolysis of peptide bonds – denaturing agents • heat • organic solvents • Urea • guanidinium chloride • detergents – SDS • change in pH – strong acids or bases • Heavy metals – Pb or Hg – Reducing agents • Beta mercaptoethanol – reduces disulfide bonds