Electrophilic Aromatic Substitution WWU-Chemistry
advertisement

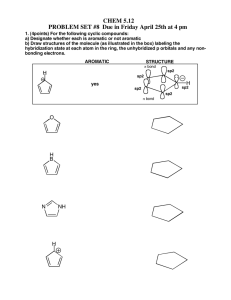
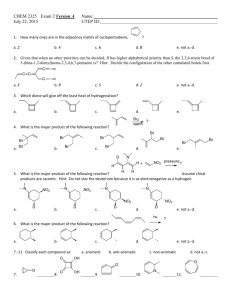
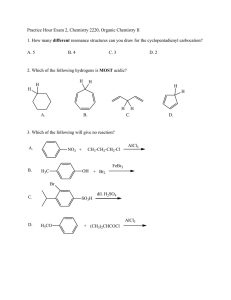
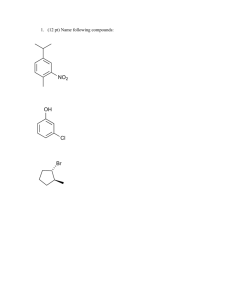
Electrophilic Aromatic Substitution WWU-Chemistry Sect. 22.1 Nomenclature CH 3 benzene Cl chlorobenzene toluene aniline NO 2 OH nitrobenzene phenol O O NH 2 OH benzoic acid H benzaldehyde WWU-Chemistry Nomenclature-- examples Group ortho or 2 meta or 3 para or 4 O O H OH Cl NO 2 3-nitro benzoic acid (IUP AC) m-nitrobenzoic acid (common) 4-chlorobenzaldehyde (IUPAC) p-chlorobenzaldehyde (common) WWU-Chemistry Chapter 22: Aromatic Substitutions, Monosubstitution reactions on benzene Sect. 22.2 Electrophilic aromatic substitution mechanism Sect. 22.5 Nitration Sect. 22.6. Halogenation Sect. 22.7 Friedel-Crafts Reactions Sect. 22.8 Sulfonation Reactions (skip fall 2006) WWU-Chemistry Sect. 22.2 Electrophilic aromatic substitution. H E+ H H H SLOW E E E resonance stabilized cation E + HB H re-aromatize E :B "base" delocalized cation WWU-Chemistry Sect. 22.5: Nitration • conc. HNO3 and H2SO4 react to make electrophile, NO2+ • nitro aromatics are important intermediates • reduction of nitro groups give anilines HNO3 NO2 + H2O H2SO4 HSO4 H2SO4 H NO2 NO2 O N O nitronium ion NO2 reduce NH2 reducing agents: LiAlH4 or H2 / Pd(C) or Sn / HCl or Fe / HOAc WWU-Chemistry Mechanism of Aromatic Nitration Step 1: Where does the electrophile come from? H O HO + N H2SO4 O O H N O O H H HSO4 O O N O N O O + H O H Nitronium ion Nitronium ion (NO2+) is the electrophile that reacts with the benzene ring. WWU-Chemistry Mechanism of Aromatic Nitration (Step 2) H + NO2 H slow H NO2 NO2 H NO2 a resonance-stabilized arenium ion WWU-Chemistry Mechanism of Aromatic Nitration (Step 3) H NO2 fast + NO2 + HSO4 H2SO4 this represents the resonance hybrid of the arenium ion H O O -S NO2 O NO2 OH O H-O S OH O WWU-Chemistry Sect. 22.6: Halogenation • active electrophile is a bromonium or chloronium ion • need Lewis acid catalyst ( FeX3 ) to activate X2 Br Br2 + HBr FeBr3 mechanism: Br2 + FeBr3 Br + FeBr4 Br HBr H Br Br Br bomonium ion Cl2 Cl + HCl FeCl3 (iodination requires special conditions) WWU-Chemistry Sect. 22.7: Friedel-Crafts Alkylation • alkyl halide + AlCl3 -->carbocation + AlCl3X• watch out for carbocation rearrangements! • more than one alkylation can occur --> mixtures! + + AlCl3 (cat) Br CH2Cl2 AlCl3 (cat) Br CH2Cl2 HBr + + HBr WWU-Chemistry Friedel-Crafts Acylation • • • • acid chloride + AlCl3 --> acylium ion + AlCl4cation rearrangements are NOT observed! acylation will only occur ONCE... reaction VERY sensitive to substituents-- an acyl group prevents further reaction O O AlCl3 (cat) + + HCl Cl O AlCl3 O C O Cl AlCl4 an acylium ion WWU-Chemistry H O H Two other resonance structures O _ H+ O WWU-Chemistry Aromatic substitution on Benzene • Sect. 22.8: • Sect. 22.9: Sulfonation (skip, fall 06) Summary WWU-Chemistry Sect. 22.10 and 22.11: Directing effects • methoxy group releases electrons by resonance effect: ortho and para director • nitro group withdraws electrons by inductive and resonance effect: meta director WWU-Chemistry These are ortho and para directors! All are electron releasing!! .. -O-H .. .. -O-R .. O .. -O-C-R .. .. -NR2 -R .. . -X .. . R = H or alkyl O .. -N-C-R H WWU-Chemistry All ortho/para directing groups have pairs of electrons next to the benzene ring! The only exception are alkyl groups. They are also ortho/para directors. WWU-Chemistry Why do ortho/para groups direct as they do? Resonance!! O O H3C AlCl3 Cl H AlCl4 H3C O CH 3 O O CH 3 CH 3 H CH 3 _ O CH 3 CH 3 H O O Meta won't work! can't put + next to O-CH3 O H O CH 3 CH 3 H O CH 3 Extra resonance structure from lone pair on oxygen! Ortho would work CH 3 too. O WWU-Chemistry These are meta directors! All are electron withdrawing!! O O R CH 3 N CH 3 CH 3 O H F C F F O O-R O N O NH 2 O S OH O C N Notice! These groups have electronegative elements next to the benzene ring! There are NO non-bonded electrons next to the ring! WWU-Chemistry Now let’s look at a meta directing group O O C CH3 C H2SO4 O + H HNO3 CH3 O + 15°C H 2O NO2 This is an example of Electrophilic Aromatic Substitution (EAS). WWU-Chemistry Why does the nitration reaction take place preferentially at the meta position? Let’s ask a “what if” question. WWU-Chemistry O OCH3 O OCH3 C H O H NO2 OCH3 O C BAD! NO2 O OCH3 OCH3 C C O H H H NO2 NO2 NO2 OCH3 O C C H OCH3 H NO2 para C C ortho meta O NO2 H OCH3 O OCH3 C BAD! NO2 H NO2 WWU-Chemistry meta substitution preferred because the + charge is never next to the CO2R group WWU-Chemistry Activation during substitution All ortho and para directing groups are activating relative to benzene, except halogen substituents. Halogens are weakly deactivating but are still o, pdirectors. G Electron releasing groups (G) help stabilize the + in the ring introduced by the electrophile. This increases the rate of substitution! + H E WWU-Chemistry Deactivation during substitution • All meta directors are strongly deactivating relative to benzene. G Electron withdrawing groups (G) intensify the + in the ring introduced by the electrophile. This slows the substitution reaction! + H E WWU-Chemistry Sect. 22.12 and 22.17: Some synthetic examples involving aromatic substitution WWU-Chemistry • ortho/para directors can work together with meta directors. They reinforce each other. O-CH 3 O-CH 3 HNO 3 HNO 3 H2SO4 H2SO4 NO 2 ortho/ para director O-CH 3 NO 2 NO 2 ortho/para meta WWU-Chemistry Strong o/ p directors win over weak o, p and meta directors. OH OH Br Br2 CH 3 FeBr3 CH 3 WWU-Chemistry Substitution RARELY occurs in-between two substituents--too hindered! O CH 3 nothing here! CH 3 H3C Cl CH 3 AlCl3 CH 3 O CH 3 WWU-Chemistry Some groups can be modified to change their directing effects. O CH 3 OH KMnO 4 Br2 ortho/para director meta director Br2 FeBr3 FeBr3 CH 3 CH 3 Br O OH Br KMnO 4 O OH KMnO 4 O OH Br 3-bromobenzoic acid Br 2-bromobenzoic acid Br 4-bromobenzoic acid WWU-Chemistry Good stuff! Order of reaction is critical! CH 3 O OH KMnO 4 HNO 3 H2SO4 HNO 3 H2SO4 CH 3 CH 3 NO 2 KMnO 4 O OH NO 2 KMnO 4 O OH O OH NO 2 NO 2 2-nitrobenzoic acid NO 2 4-nitrobenzoic acid 3-nitrobenzoic acid WWU-Chemistry Some more good stuff! O H3C O Cl O CH 3 CH 3 Br2 FeBr3 AlCl3 Br meta director O Br2 O CH 3 H3C FeBr3 Cl Br ortho/para director AlCl3 Br WWU-Chemistry An explosive! CH 3 O2N NO 2 NO 2 2,4,6-trinitrotoluene (TNT) WWU-Chemistry CH 3 CH 3 CH 3 CH 3 HNO 3 H2SO4 Easy! NO 2 HNO 3 NO 2 H2SO4 CH 3 NO 2 more difficult Heat it! HNO 3 O2N NO 2 H2SO4 NO 2 NO 2 Even more difficult so really heat it! 2,4,6-Trinitrotoluene = TNT WWU-Chemistry Some miscellaneous examples • • • • Nitration of 3-nitrobenzoic acid Acylation of 1,3-dimethylbenzene Acylation of 1,4-dimethylbenzene Make 2-methyl-1-phenylpropane WWU-Chemistry Sect. 22.16 Aromaticity and Huckel’s Rule Aromatic compounds 4n + 2 pi electrons n=1 6 pi electrons systems + Cation : _ Anion WWU-Chemistry Empty p-orbital H H 6-pi electrons This is a n = 1 with 6 pi electrons H Aromatic! WWU-Chemistry Other n = 1 aromatics H : N 6 electrons : : S 6 electrons, one pair not involved! : : O 6 electrons, one pair not involved! All are aromatic! WWU-Chemistry rehydridize the oxygen 3 2 atom from sp to sp O Oxygen has two pairs, but only one pair is in the pi system (p-orbital). This system is a n = 1 system, with 6 pi electrons This compound is AROMATIC! WWU-Chemistry n = 0 aromatic: 2 pi electrons + WWU-Chemistry H H 2 pi electrons Aromatic! H H H H Cyclopropene is not aromatic, you need p orbital at all positions! H WWU-Chemistry Some Antiaromatic compounds : _ 4 electrons 4 electrons 8 electrons Not aromatic! WWU-Chemistry Diazonium ions, Azo Dyes and the Sandmeyer Reaction- from Chapter 23 (not covered 06) Sect 23.16: Diazonium ion formation Sect 23.17: Sandmeyer reaction Sect 23.19: Azo dyes WWU-Chemistry