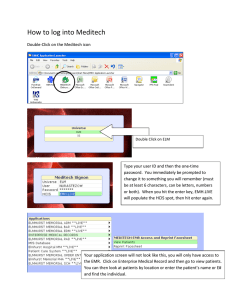
TULANE HOSPITAL AND CLINIC INVESTIGATIONAL STUDY QUESTIONAIRE STUDY TITLE:
advertisement

TULANE HOSPITAL AND CLINIC INVESTIGATIONAL STUDY QUESTIONAIRE STUDY TITLE: This form is filled out, along with the Meditech Charge Form, and is brought to the Monday 9:00 am meetings to discuss the study and have Finance agree to the charges PRIOR TO IRB SUBMISSION. Principle Investigator: Study Coordinator: E-mail: Phone: Department: Sponsor: PLEASE COMPLETE THE FOLLOWING QUESTIONS 1. Indicate the date you anticipate the study to begin: Expiration date: 2. Please indicate if this a Medical Record Review study only: If yes, complete line 12 only and have PI sign form. After Quality Management approves the study, this form and a signed Electronic Medical Record (EMR)/Paper Medical Record request form is then brought to Health Information Management (HIM) for access to Medical Records. 3. 4. 5. 6. Anticipated number enrolled each month? Gender and age range: Do you plan to enroll Medicaid Patients? Please specify exact locations where study will be conducted TUHC Inpatient TUHC Outpatient Surgery billing request form is required for Outpatient Surgery 7. What type of space will be utilized? e.g., exam room, consultation/procedure, hospital bed, recovery room, etc. **TWO MEDITECH CHARGE FORMS ARE REQUIRED** All cost/charges not billed to patient/insurance as medically necessary must be documented on a Meditech Charge Form. All Standard of Care charges must be documented on a separate Meditech Charge Form. Medicaid/Medicare requires TUHC to submit the 8 digit Clinical Trial Number with ALL services rendered during a study. You can get this number at WWW.CLINICALTRIALS.GOV. 8. What additional TUHC assistance/services will be needed? e.g., Specimen prep, anatomic pathology, blood collection, etc. 9. Who will provide training to the TUHC staff? 10. When will the training be conducted? 1 TULANE HOSPITAL AND CLINIC INVESTIGATIONAL STUDY QUESTIONAIRE 11. Have you met with unit/clinic/outpatient/ancillary department leaders to discuss the implementation of this study? (i.e., Procedures, supplies, equipment or training). List names of Department Directors/Managers you have discussed requirements with. 12. List the names & titles of all persons who will be involved in performing the study (please identify any physicians, nurses, research coordinators, fellows, etc. who are not TMC employees). *All study coordinators must be credentialed. You may only provide study participant care within your scope of practice. For credentialing, please contact TMC’s Human Resources. 13. Will investigational drugs be used? a. Have you contacted the Clinical Research Pharmacist? b. Where will the drugs be stored? 14. Will any electrical or battery medical equipment not belonging to TUHC be utilized? In accordance with the Joint Commission Environment of Care Standard EC 1. 6, all medical equipment entering TUHC (this includes satellite clinics), regardless of ownership (physician owned, sponsor owned, loaner, etc.) must have an incoming safety inspection and evaluation. Contact TMC BioMed department at 504-988-3064. 15. Will investigational devices, instruments, or machines be utilized? a. Who will provide device/instrument/machine? b. Specify type of device/instrument/machine: c. Who will be using this equipment? Make: Model: Serial #: How will TUHC be reimbursed for treatment of an adverse reaction? Sponsor: Department: ___________________________________ PI Signature ________________________________ Quality Manager/IRB Coordinator 2