ORAL HISTORIES AND HUMAN SUBJECTS RESEARCH PROTECTION REQUIREMENTS April 18, 2012
advertisement

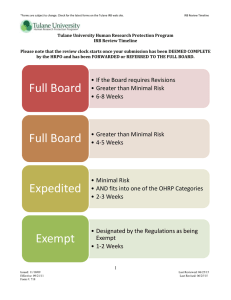
ORAL HISTORIES AND HUMAN SUBJECTS RESEARCH PROTECTION REQUIREMENTS April 18, 2012 Objectives of Presentation Better understand when oral histories are subject to Federal human subjects protection requirements Review of Tulane policies with regard to when oral histories are subject to exempt or expedited review The Challenge Is ORAL HISTORY considered “research involving human subjects”? Who decides? Why does it matter if oral history interviews meet the definition of human subject research? If not, then the activity is not subject to a myriad of federal human subject protection regulations, which Tulane is required to follow for all human subjects research If so, then the activity is subject to federal human subject protection regulations and must undergo: Convened review by Tulane’s IRB (i.e., full board review), Exempt review, or Expedited review 2-Step process for review of oral histories Step 1: Does the activity constitute human subjects research; and Step 2: If yes, does the research qualify for exempt review or expedited review Step 1 Regulatory Definition of “Research” A decision whether oral history or other activities solely consisting of open ended qualitative type interviews are subject to the policies and regulations outlined in an institution’s FWA and DHHS regulations for the protection of human research subjects (45 CFR 46) is based on: The prospective intent of the investigator; and The definition of “research” under DHHS regulations at 45 CFR 46.102(d) as A “systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.” Lack of Guidance Federal regulations do not provide a definition for: “systematic investigation” “designed” “generalizable knowledge” The regulations fail to say who is to make that determination Such decisions are made on a case-by-case basis depending upon the facts Practical Interpretations A “systematic investigation” is an activity that involves a prospective research plan that incorporates data collection, either quantitative or qualitative, and data analysis to answer a research question “Generalizable knowledge” involves studies that are designed to draw general conclusions (i.e., knowledge gained from a study may be applied to populations outside the specific study population), inform policy, or generalize findings Human Subject Research Determination The university has charged the IRB with monitoring all human subject research occurring at the university. The PI is responsible for the initial determination of whether the planned activity comprises human subjects research (Tulane SOPs 3.3) The sponsor and Tulane hold the PI accountable for wrong determinations. For this reason, PIs are strongly encouraged to request confirmation from Tulane’s HRPO that the activity does not constitute human subjects research. Requests to HRPO can be made via e-mail at irbmain@tulane.edu REMINDER: The conduct of a research project without the appropriate review is a violation of Tulane policy and Federal regulations and subject to disciplinary action. Faculty and students should err on the side of caution and contact the HRPO Office via e-mail at irbmain@tulane.edu for guidance before commencing their research. Why does it matter if human subject research is involved? Possible repercussions for failure to obtain IRB approval of human subjects research: Prohibition from using data; Prohibition from publishing; Reporting to federal oversight agencies (ex: FDA, OHRP; OIG); Subject to filing of a research misconduct allegation, which can lead to a range of penalties, including expulsion. What is oral history? There is no Federal definition of “oral history” Oral history activities in general are designed to create a record of specific historical events and, as such, are not intended to contribute to generalizable knowledge Oral history narrators are not anonymous individuals selected as part of a random sample for the purposes of a survey. Interviewees are selected because of their personal relationship to the topic under investigation. An oral history interview provides one person’s unique perspective. A series of oral history interviews offers up a number of particular, individual perspectives on the topic, not information that may be generalized to all participants in the event or time under investigation. What is oral history? (cont) Oral history interviews are not analyzed as qualitative data is generally analyzed. No content analysis, discourse analysis, coding for themes or other qualitative analysis methods of data analysis are performed on the interviews. They stand alone as unique perspectives. It depends upon the facts & investigator’s intent It is primarily on the grounds that oral history interviews, in general, are not systematic investigations designed to contribute to generalizable knowledge and are not subject to the requirements of 45 CFR part 46 and, therefore, can be excluded from IRB review. However, some oral history interviewing projects may meet the definition of research and will require convened IRB review, expedited review, or exempt review General principles for evaluating oral history type activities Open Ended Interviews Oral history activities, such as open ended interviews, that ONLY document a specific historical event or the experiences of individuals without an intent to draw conclusions or generalize findings would NOT constitute “research” as defined by DHHS regulations 45 CFR part 46 Example 1: Video Interviews Question: Is an oral history video recording of interviews with holocaust survivors created to preserve or describe individual experiences to be viewed at the Holocaust Museum considered “research” as defined by DHHS? Answer: The creation of the video tape does NOT intend to draw conclusions, inform policy, or generalize findings. The sole purpose is to create a historical record of specific personal events and experiences related to the Holocaust and provide a venue for Holocaust survivors to tell their stories. Thus, no institutional review is required Example 2: Open Ended Interviews to Document Experiences & Draw Conclusions Question: Is an oral history that involves interviews of Iraq War Veterans that is intended to draw general conclusions and otherwise learn about the impact of using National Guard soldiers in a war considered “research” as defined by DHHS? Answer: Yes, as it is designed to develop or contribute to generalizable knowledge (e.g., designed to draw conclusions, inform policy, or generalize findings) and WOULD constitute “research” as defined by DHHS regulations at 45 CFR part 46 Yes, needs review. The investigator would have to submit an initial application to the IRB for either convened, exempt or expedited review Example 3: Interviews for Publications Question: Does interviewing prisoners for a magazine article on prison life constitute human subjects research? Answer: No. The goal is not the production of generalizable or universal knowledge. Rather, the information generated is specific to the people interviewed in their current situation. Thus, no institutional review is required Example 4: Oral Histories for Archival Purposes Question: Whether open ended interviews conducted with surviving Negro League Baseball players intended to create an archive for future research constitutes research? Answer: Yes. The creation of such an archive would constitute research under 45 CFR part 46 since the intent is to collect data for future research. Since the intent of the archive is to create a repository of information for other investigators to conduct research as defined by 45 CFR part 46, the creation of such an archive WOULD constitute research under 45 CFR part 46. The investigator would have to submit an initial application to the IRB for either convened, exempt or expedited review What is exempt research? Minimal risk studies that fall within set categories listed in Tulane SOPs 3.4.2. with a shortened IRB application. While exempt research is human subject research requiring institutional review, it does not require convened (i.e., full) IRB review. Such review is typically approved by the IRB chair (or designee) One category of exempt research potentially applicable to oral histories is research involving the use of educational tests, survey procedures, interview procedures, or observation of public behavior, unless: Information obtained is recorded in such a manner that Human Subjects can be identified directly or through identifiers linked to the subjects; and Any disclosure of the Human Subjects responses outside the research could reasonably place the subjects at risk Exempt research can never include research involving children, prisoners or that is international in nature [Tulane SOPs 3.4.1] If planned oral research is not exempt, consider whether it qualifies for expedited review What is expedited research? Requires one or more experienced IRB reviewers, but does not require a convened IRB [see Tulane SOPs 3.5] There must be no more than minimal risk to subjects. The identification of participants will not place them at risk of criminal or civil liability or be otherwise damaging Review is conducted by the IRB Chair or designee and approval period is for up to one year. Categories of expedited research The research must fit into one of several specific categories enumerated in Tulane SOP 3.5.1to qualify as expedited research. Common categories include: Category 6 Category 7 Collection of data from voice, video, digital, or image recordings made for research purposes Research on individual or group characteristics or behavior (including research on perception, cognition, motivation, identify, language, cultural beliefs, social behavior, etc.) If planned oral research does not qualify for exempt or expedited review, then it must go to full board review. Summary If you wish to avoid triggering federal human subjects research protection regulations, try to structure activity to avoid definition of human subjects research, which would eliminate the need for IRB review Alternatively, meet the definition of exempt review Alternatively, fit research into an expedited review category Benefits: shortened initial application, may be granted up to 3 years exempt IRB approval, and review by IRB chair (or designee) rather than full board Benefits: review by IRB chair (or designee) rather than full board review If you have questions, please contact Tulane’s Office of Human Research Protection (HRPO) at 504-988-2665 or by E-mail at irbmain@tulane.edu