IS THERE A BENEFIT OF SEXUAL SELECTION IN A COMPETITIVE
ENVIRONMENT IN DROSOPHILA MELANOGASTER?
Yukiharu Miyashige
B.S., California State University, Sacramento, 2007
THESIS
Submitted in partial satisfaction of
the requirements for the degree of
MASTER OF SCIENCE
in
BIOLOGICAL SCIENCES
at
CALIFORNIA STATE UNIVERSITY, SACRAMENTO
SPRING
2012
© 2012
Yukiharu Miyashige
ALL RIGHTS RESERVED
ii
IS THERE A BENEFIT OF SEXUAL SELECTION IN A COMPETITIVE
ENVIRONMENT IN DROSOPHILA MELANOGASTER?
A Thesis
by
Yukiharu Miyashige
Approved by:
__________________________________, Committee Chair
Brett Holland, Ph.D.
__________________________________, Second Reader
Ronald M. Coleman, Ph.D.
__________________________________, Third Reader
Thomas R. Peavy, Ph.D.
____________________________
Date
iii
Student: Yukiharu Miyashige
I certify that this student has met the requirements for format contained in the
University format manual, and that this thesis is suitable for shelving in the Library
and credit is to be awarded for the thesis.
______________________________, Graduate Coordinator
Ronald M. Coleman, Ph.D.
Department of Biological Sciences
iv
________________
Date
Abstract
of
IS THERE A BENEFIT OF SEXUAL SELECTION IN A COMPETITIVE
ENVIRONMENT IN DROSOPHILA MELANOGASTER?
by
Yukiharu Miyashige
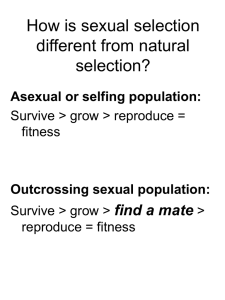
Charles Darwin introduced the concept of intersexual selection suggesting that
apparently costly conspicuous male secondary sexual traits have evolved because they
aid individuals in obtaining mates, even at the cost of male survival.
Since Darwin’s time, considerable progress has been made in the study of
sexual selection. The good genes hypothesis has been one of the major interests of
researchers, and a significant amount of empirical research has been conducted in
Drosophila melanogaster. The hypothesis states that individuals of one sex (usually
female) prefer specific reproductive partners because preferred mates would bring
greater genetic quality to offspring than random mates.
In this experiment, populations (n=3) of D. melanogaster experiencing sexual
selection (promiscuous), or its absence (monogamous), were allowed to evolve against
a non-coevolving competing population. If the benefit of sexual selection exceeds the
cost under this competitive environment, then we should observe the evolution of
higher fitness in promiscuous populations, and it would imply that sexual selection is
v
adaptive with respect to larval competition. Hence, the good genes hypothesis would
be supported.
After 16 generations of selection under the competitive environment, I did not
observe a measurable adaptation with the presence of sexual selection. There was no
significant difference in the fitness between promiscuous and monogamous
populations. While the promiscuous populations had higher measured fitness due to
apparently greater development rate, this result is inconclusive because of the
systematic difference in density over the course of the experiment. Repetition of this
research would require stricter control on population density.
______________________________, Committee Chair
Brett Holland, Ph.D.
_______________________
Date
vi
ACKNOWLEDGEMENTS
Dr. Brett Holland taught me everything. If I had not met him, I could not be the
person I am now. He made me a better person, and I cannot thank him enough.
Without the guidance and expertise of Dr. Ronald Coleman, Dr. Thomas
Peavy, Dr. Jamie Kneitel, and Dr. Nicholas Ewing, I could not have finished this
thesis. From all my professors, I have learned not only how to observe and learn about
the nature, but also what it is to be a scientist. I was not the best student of theirs, but
they have been always patient with my progress and they have always encouraged me
to go through my graduate program. I sincerely appreciate everything they have
provided for me.
My fellow students Mr. Colin Contino and Mr. Larry Cabral have provided me
significant assistance and insight for my graduate student life both inside and outside
the school. I could not have finished my graduate program without them.
Mr. Eric Merchant and Ms. Tracey Culbertson from the Office of Global
Education at CSUS have provided me a great amount of support in order for me to
stay in the U.S. as an international student. Without their help, I could not have
finished my education at this university.
Lastly, I thank all my friends I made in the United States of America. When I
first came here, I did not have any friends and I could not even speak English at all. I
had nothing. They taught me how to communicate in English and how to live in the
U.S. Without them, I could not survive, enjoy my life, and finish my education.
vii
TABLE OF CONTENTS
Page
ACKNOWLEDGEMENTS ....................................................................................... vii
LIST OF TABLES ..................................................................................................... ix
LIST OF FIGURES ..................................................................................................... x
INTRODUCTION ....................................................................................................... 1
MATERIALS AND METHODS ............................................................................... 10
RESULTS .................................................................................................................. 21
DISCUSSION ............................................................................................................ 36
CONCLUSIONS ........................................................................................................ 39
LITERATURE CITED .............................................................................................. 40
viii
LIST OF TABLES
Tables
Page
1.
Standard culturing conditions ..........................................................................11
2.
Procedure of fitness assay ................................................................................16
3.
Regression Analysis for fitness and productivity with respect
to the first 16 generations of experiment .........................................................22
4.
Test for the homogeneity-of-regression for fitness, productivity,
and vial density ................................................................................................23
5.
ANCOVA of treatment and generation with respect to vial density ...............26
6.
Student’s t-test for low-competition development assay .................................30
7.
Student’s t-test for high-competition development assay ................................32
8.
Student’s t-test for inbreeding assay ................................................................34
ix
LIST OF FIGURES
Figures
Page
1.
Mating cartridges for treatments ......................................................................15
2.
Fitness of promiscuous and monogamous populations ...................................24
3.
Productivity of promiscuous and monogamous populations ...........................25
4.
Vial density ......................................................................................................27
5.
Rate of development with low competition .....................................................31
6.
Rate of development with high competition ....................................................33
7.
Degree of inbreeding depression .....................................................................35
x
1
INTRODUCTION
Charles Darwin (1871) first introduced the concept of intersexual selection to
explain the evolution of apparently costly conspicuous male secondary sexual traits, such
as the colorful ornaments and displays that would significantly decrease males’ chances
of survival. He suggested that these sexual traits have evolved because they aid
individuals in obtaining mates, even at the cost of male survival. Darwin’s explanation of
sexual selection was remarkably insightful given the preliminary stage of evolutionary
biology in the 19th century, and many of his original thoughts are still valid in modern
sexual selection research (Jones and Ratterman, 2009). However, Darwin’s original
sexual selection model was only qualitatively related to reproductive success. After “the
genetic theory of natural selection” was introduced by Ronald Fisher (1930), sexual
selection was tied to fitness. The current definition for sexual selection is “the nature and
consequences of competition over mates” (Andersson, 1994) which creates “differences
among individuals of a sex, in the number or reproductive capacity of mates they obtain”
(Futuyma, 2005).
Since Darwin’s time, considerable progress has been made in the study of sexual
selection. Especially in the last several decades, evolutionary biologists have extensively
studied how the preference of one sex for the specific secondary sexual traits of the other
sex has evolved. The understanding of mating preference is the current key topic of
sexual selection. Once we understand how mate preference has evolved, then the
2
evolution of preferred sexual traits would be explained (Jones and Ratterman, 2009;
Møller and Alatalo, 1999). The purpose of my research is to understand how mate
preference has evolved. More specifically, I hypothesize that individuals of one sex
(usually female) prefer specific reproductive partners because preferred mates would
bring greater genetic quality to offspring than random mates. A corollary of this is that
sexual selection will increase evolutionary fitness of a population.
The reason why females are choosier than males about their mates was suggested
by Angus Bateman (1948). Bateman empirically demonstrated that male fruit flies,
Drosophila melanogaster, can increase their lifetime reproductive output by increasing
the number of their mates, whereas females can not increase their lifetime reproductive
output regardless of the number of mates. This finding indicated that the optimal mating
rate of males is higher than that of females, and so reproductive behavior would evolve
differently between sexes within a species. Robert Trivers (1972) then pointed out the
difference of parental investment between two sexes. In most sexually reproducing
species, female parental investment (e.g., large nutrient-rich gametes, internal
development, and postpartum care) is far greater than that of males. Males generally
invest in fertilization success. Most obviously, males invest in producing far more,
smaller, motile gametes to maximize fertilization success rather than investing in
resources in the growth and development of their offspring. This basic difference is
rooted in anisogamy, and it has been suggested that once the sexes specialized at the
3
gametic level (between nutrient rich gametes and small motile gametes), further sexual
dimorphism, including behavior, was inevitable (Chapman et al., 2003).
Models for the evolution of mating preference can be categorized into two major
groups: direct-benefits models and indirect-benefits models. The direct-benefits models
explain that females (or members of a sex which provides greater parental investment to
their offspring than the other sex) choose mates who provide immediate benefits, such as
a nuptial gift, territory defense, or parental care (Kokko et al., 2003). The concept of
direct-benefits models is simple and not controversial, and the presence of direct benefits
between sexes in various species is well documented, both theoretically and empirically
(Kirkpatrick and Ryan, 1991; Kirkpatrick, 1996; Iwasa and Pomiankowski, 1999; Kokko
et al., 2003; Kotiaho and Puurtinen, 2007). However, direct-benefits models become less
applicable when females prefer male sexual ornaments in species in which males do not
provide direct benefits (Jones and Ratterman, 2009). In fact, in most species with
prominent male ornaments, including the model organism of my experiment (Drosophila
melanogaster), males do not provide direct benefit to females (Kirkpatrick and Ryan,
1991; Kokko et al., 2003).
Hence the second group of models, indirect-benefit models, explains that female
mating preference has evolved because mating with a preferred mate may result in higher
fitness of offspring through genetic qualities inherited from their fathers. Among the
indirect-models is Fisher’s (1930) runaway hypothesis. In this model, females who prefer
4
males with an ornament produce sons that have the ornament and daughters that have the
preference. Females who lack the preference mate with males who lack the ornament and
they produce daughters and sons of the same phenotype, respectively. If for any reason
the preference and the ornament increase to some threshold frequency, positive feedback
makes the process self-reinforcing and then sons who lack the ornament are selected
against (Andersson, 1994).
Fisher’s runaway hypothesis may explain why females would mate polygynously.
Weatherhead and Robertson (1979) proposed the sexy son hypothesis, inspired by work
on polygynous blackbirds, Agelaius phoeniceus (Orians, 1961). The authors argued that
females evolve a preference for males who are attractive but may offer inferior resources
and, as a result, fewer offspring. The direct cost to polygynous females of lower
fecundity is compensated in the second generation when her sexy sons, who have
inherited their father’s genes and hence his ability to attract mates, sire more
grandchildren than the sons of monogamous females.
Finally, ornaments may indicate the bearer's genetic quality outside of the context
of sexual selection (i.e., good genes hypothesis) (Fisher, 1930; Pruutinen et al., 2009).
The key to the good genes hypothesis is that the ornament is expensive in proportion to
its magnitude (e.g., increased energy expenditure or exposure to predators). Males with
inferior genomes who make a large ornament would suffer, while males who can thrive
despite the large ornament will have demonstrated to females that they can compensate
5
for the handicap, thereby indicating superior genetic quality (Zahavi, 1975). If this
quality is also heritable then females can indirectly increase their fitness by mating with
males who have the largest ornament, because their sons and daughters will also be
genetically superior. Therefore, females can be expected to have more or better grand
children (Fisher, 1915; Williams, 1966).
The evolutionary process of self-reinforcing coupling between sexual traits and
preference hypothesized by the good genes hypothesis is almost identical to that of the
sexy son hypothesis (Kokko et al., 2002). The difference is that the good genes theory
explains that females indirectly increase their fitness by producing offspring (not only
sons but also daughters) that have higher viability, while the sexy son hypothesis
explains that females do so only through the enhanced mating success of male offspring
(Cameron et al., 2003; Stewart, 2005).
The good genes hypothesis is where the vast majority of empirical research has
been conducted (for reviews, see Pruutinen et al., 2009; Kokko et al., 2006; Mead and
Arnold, 2004). Perhaps the most convincing test was conducted by Welch et al. (1998)
with grey tree frogs, Hyla versicolor. They took sperm from long-calling and shortcalling males, and fertilized half of a single female's eggs with each type of sperm
(thereby controlling for differences between females). Then, they reared the offspring in
two types of artificial ponds (one with a high amount of food, and the other with a low
amount of food), and measured 5 components of offspring fitness. Some components
6
were superior in the offspring of the long-calling males in one or both types of artificial
ponds, and none were superior in the offspring of short-calling males. Thus they
concluded that call duration is in fact a reliable indicator of heritable genetic quality in
males.
In Drosophila melanogaster, Partridge (1980) first showed that females could
increase the fitness of their offspring through mate choice. She made replicate
populations (n=2) of females and allowed half of the populations to experience sexual
selection (100 females housed with 300 males). Alternatively, 100 females were placed
into individual containers, each with only one male, thereby enforcing random,
monogamous mating with no possibility for sexual selection (female choice or male-male
contests). The larval offspring from each treatment were reared in competition with
offspring of the same age that had a visible mutation (marker). She observed that the
populations in which sexual selection was allowed had more survivors (larva-to-adult
viability) than the monogamous populations, as predicted by the good genes hypothesis.
However there are also similar studies where the benefit of sexual selection was
not found. Schaeffer et al. (1984) reported that Partridge’s (1980) experiment was not
repeatable with either D. melanogaster and D. pseudoobscura, they found no association
between sexual selection and offspring viability. Promislow et al. (1998) increased the
power of Partridge’s (1980) experiment by repeating the mating treatment for nine
7
generations, and found no significant increase of offspring viability in promiscuous
populations compared to monogamous populations.
Holland (2002) also investigated the benefit of sexual selection with a similar
design. In his experiment, he further increased the experimental power by applying an
abiotic environmental stress (a thermal stress) and by adding the duration of mating
treatment (36 generations). In spite of the greater experimental power, no benefit of
sexual selection was found in his experiment either.
Gowaty et al. (2010) recently reported that sexual selection enhanced female
fitness in Drosophila pseudoobscura. Similar to Partridge's experiment, they varied the
degree of sexual selection by creating three different mating treatments: monogamous
mating with single copulation, monogamous mating with multiple copulations, and
polyandrous mating with multiple copulations. They measured the number of adult
offspring (i.e., productivity) and egg-to-adult survival (i.e., offspring viability) as fitness
measures of female parents. They also measured maternal lifespan in order to assess the
cost of polyandrous mating (e.g., harmful seminal fluid protein) as reported by previous
studies by Moore et al. (2003). They found that productivity and offspring viability from
females with polygamous mating were the highest, while there was no significant cost of
polyandrous mating compared with monogamous mating. Their finding clearly supports
that the presence of sexual selection was beneficial to the population of Drosophila
pseudoobscura in their experimental environment.
8
Collectively, the benefit of sexual selection is inconclusive in D. melanogaster.
Most experiments I have reviewed measured the population fitness under the standard
laboratory condition which is simplistic compared to nature. Thus they may not have
observed an important benefit of sexual selection through the good genes process mainly
due to their low experimental power. Holland’s experiment (2002) was novel in that it
measured adaptive benefit of sexual selection under an abiotic environmental stress
(thermal stress). However, in nature, a population is simultaneously under viability
selection not only caused by abiotic environmental factors (Bettencourt et al., 2002) but
also by biotic factors such as interactions with other species.
The purpose of my research was to measure the adaptive benefit of sexual
selection under the stress of biotic environmental factors, that is competition for
resources. One source of chronic selection is intense larval competition while developing
on ephemeral rotting fruit. More specifically, populations experiencing sexual selection,
or its absence, are allowed to evolve against a non coevolving competing population as
would occur in nature, if a novel monogamy mutant were to arise within a population. In
my experiment, I allowed both monogamous and promiscuous (sexually-selected)
populations of D. melanogaster to each develop with a non-coevolving common
competitor population (white-eyed mutant D. melanogaster), and measured the relative
fitness of each promiscuous and monogamous population against the white-eyed
population over evolutionary time. In order to prevent the competitor population from
9
adapting to the treatment populations, the white-eye population was taken from a naive
stock population. If the benefit of sexual selection exceeds the cost under the given
environment, then I should observe the evolution of higher fitness in promiscuous
populations, and it would imply that sexual selection is adaptive with respect to larval
competition, hence the good genes hypothesis would be supported. On the other hand, if
I do not observe the net benefit through sexual selection (i.e., monogamous populations
evolve higher fitness), then different mechanisms of sexual selection, such as sexual
conflict models (Parker 1976, 2006), need to be considered.
10
MATERIALS AND METHODS
Populations of Drosophila melanogaster
Two populations of D. melanogaster were provided from the laboratory of Dr. W.
R. Rice at The University of California, Santa Barbara. One was from the LHM line (wild
type), and the other was from the LHM-w line ("white-eye” mutant that has been
introgressed into the LHM background). LHM population has adapted to the laboratory
condition for approximately 500 generations and has been maintained at a large
population size (>5000). The population was established by Harshman from 400 mated
females collected in central California in 1988, and has been maintained in the standard
condition described below. LHM-w was derived from LHM approximately four years ago.
Fifty-six culturing vials from LHM population and 14 culturing vials from LHM-w
were received on March 5th, 2009, with each vial containing 16 male-female pairs (896
individuals of each sex in LHM and 224 individuals of each sex in LHM-w). Until the first
day of the experimental procedure (May 11th, 2009), these two populations were kept for
5 generations in the standard condition (Table 1). During this period, these populations
were expanded to a larger size: approximately 6000 individuals, both sexes combined,
per generation.
11
Table 1. Standard culturing conditions.
Condition
Description
Temperature
25 °C, constant.
Lighting
12-h light and 12-h dark, 24-h diurnal cycle
Humidity
Ambient
Population density
Base population: 600 ± 30 embryos per 6-oz (≈ 180
mL) container. Each container is filled with 60 mL of
food medium.
Experimental population: Specified in each assay,
described below.
Food
Molasses/killed-yeast medium, with additional live-yeast
to promote oviposition.
Generation cycle
14 days per generation without overlap.
12
Standard Laboratory Condition
All populations were kept in the conditions described in Table 1, before and
during the experimental procedure unless specified otherwise. This condition has been
established as the standard culturing condition in many studies (Ashburner et al., 2005).
Mating Cartridges and Culture Vials
Mating cartridges were crafted for mating treatments. The material used was
transparent polyethylene terephthalate (PET) tubes (inner diameter 9.0 mm, length 50.0
mm) which were assembled as shown below (Figure 1). Mating tubes of cartridges for
monogamous mating treatment were completely separated, thus no individuals could
move from one tube to another. Mating tubes of cartridges for promiscuous mating
treatment were connected by removing the top 1/3 of inner tubes, allowing all individuals
in these cartridges to interact with each other. Food medium was filled to approximately
10.0 mm from the bottom of each cartridge.
The culture vial used for culturing LHM-w population and for the oviposition
process of the experiment was a transparent polypropylene circular vial (inner diameter
26.0 mm, length 90.0 mm) with each containing 10 mL of food medium.
13
Fitness Assay
Difference in adaptation rate in fitness. At the beginning of the experiment, 342
males and 342 females were randomly chosen in order to make 3 replicate populations.
One replicate population consisted of 96 males and 96 females. 37 extra males and 37
extra females were collected and treated identically to ensure that 96 individuals of both
sexes were used for oviposition. A detailed experimental procedure is described below
(Table 2). The data used to measure the fitness of populations was the number of
successfully developed red-eyed adult individuals from the first two days of collection
period (Day 9-10) in each generation. Only adults collected on the first two days were
used to create a following generation, thus evolutionary pressure was applied mainly to
the individuals collected in this period.
Difference in adaptation rate in productivity. The same populations and
procedure described above were used. The data used to measure the productivities of
populations was the number of successfully developed red-eyed adult individuals from
entire collection period (Day 9-20) in each generation.
Verification of vial density. The number of all individuals (red-eyed + whitedeyed) from the entire collection period (Day 9-10) in each generation was compared
between treatments in order to verify if the vial density was properly standardized.
The fitness assay was started on May 27, 2009. The data was collected in each
generation from the 1st to the 16th generation. The assay was continued till the 32nd
14
generation followed by low-competition development assay, high-competition
development assay, and inbreeding assay.
Development Rate Assays
Low-competition development assay. From the 30th generation of the fitness
assay, 96 red-eyed females from each population were transferred into an embryo-laying
cage (Clear PET plastic, 120 mm x 120 mm x 215 mm). An embryo-collection circular
dish (diameter: 100 mm, depth: 15 mm) filled with embryo-collecting media was placed
inside each cage. Food media used for the embryo-collecting plate was high
concentration agar and molasses. Females were allowed to produce embryos for 8 hours.
Soon after oviposition, exactly 20 embryos were transferred to a culture vial. Each
population was consisted of 10 vials. Eclosing adults were collected and counted twice
daily at 11 a.m. and 8 p.m., from the 9th to the 13th day after oviposition.
High-competition development assay. Individuals from the 32nd generation of the
fitness assay were used for this assay. Except as noted below, the assay was identical to
the low-competition development assay. Females were allowed to lay embryos for 12
hours. Soon after oviposition, exactly 50 embryos were transferred to a culture vial. To
the same culture vial, approximately 200 embryos from the white-eyed competitor
population were added. Each population consisted of 7 vials. Eclosing adults were
15
Figure 1. Mating cartridges for treatments. A) A cartridge for the monogamous
mating treatment. Each monogamous couple is placed in a separated tube. B) A
cartridge for promiscuous mating treatment. Interaction among all individuals placed
in all tubes is allowed.
16
Table 2. Procedure of fitness assay.
Day
1
3
4
5
Treatment
Description
Starting
Treatment
At 11 a.m. of on Day 1, 19 virgin males and 19 virgin females
are transferred to treatment cartridges. In a cartridge for
monogamous mating treatment, 19 one-male-one-female pairs
are kept separately until females are transferred into oviposition
vials on Day 4. In a cartridge for promiscuous mating treatment,
19 males and 19 females are kept together, and they are
allowed to interact with each other freely until Day 4. In this
process, flies are anesthetized briefly (<3 min) with moisturized
CO2. The same amount of yeast is applied to each cartridge,
both monogamous and promiscuous mating treatment.
Flipping
Mating
Cartridge
In the morning of Day 3, all cartridges are changed to new ones
with fresh food in order to keep flies in a healthy condition.
Cartridges are changed carefully so that all monogamous pairs
are kept with the same partners, and individuals of a
promiscuous mating cartridge are also kept the same. This
flipping procedure is done in order to keep flies in a constant
fresh environment.
Oviposition
&
Female
Competition
for Resource
Clearing
Females
from
Oviposition
Vials
At 11 a.m. on Day 4, 8 females from monogamous cartridges
and 8 randomly chosen females from LHM-w base population are
put together into different oviposition vial, using minimum CO2
anesthesia (<3 min). Similarly, 8 females from promiscuous
cartridges and females from LHM-w base population are put
together into different oviposition vials. Hence each oviposition
vial contains 8 white-eye females and 8 red-eyed females from
either monogamous or promiscuous populations. CO2 exposure
is timed and constant between treatments. No additional yeast is
supplied in these oviposition vials since they do not utilize the
yeast from this point to produce embryos laid during this 24-hour
oviposition period.
At 11 a.m. on Day 5, after 24 hours of oviposition, all females in
oviposition vials are discarded, leaving behind the embryos. We
reduce the embryo density of each oviposition vials by
discarding randomly selected embryos in order to keep the total
number of embryos at about 150, which is considered to be
moderate embryo density. The vials with embryos are incubated
and kept in the standard condition described above until all
adults developed from these embryos are counted and recorded
as data for the experiment.
(continued on the next page)
17
Table 2. Procedure of fitness assay (continued).
Day Treatment
Description
9
Virgin
Collection
&
Data
Collection
At 11 a.m., adults in the vials are counted, categorized by
phenotype and by sex. Adults found at this collection period are
discarded because they may have not stayed virgin. At 7 p.m.,
newly eclosed virgin adults are counted (under <4 minutes CO2)
and transferred to new vials with fresh food. Collected virgins are
kept in the standard condition until Day 1 of the next generation.
Approximately 50% of total adults in each generation are hatched
from pupa by this point.
10
Virgin
Collection
&
Data
Collection
At 11 a.m., virgin adults in the vials are counted and transferred to
the new vials with fresh food. Collected virgins are kept in the
standard condition until Day 1 of the next generation.
14
Starting
Treatment
Treatment for the next generation is started. This is the Day 1 of
the next generation.
20
Data
Collection
Adults hatched from pupa after Day 14 are counted and discarded.
All adults in each generation are hatched from pupa by this point.
18
collected and counted once daily at noon, from days 9-13 after oviposition. Thirty two
generations of selection through fitness assay had been done prior to this assay.
Inbreeding assay
An additional 20 male 20 female virgins were collected from each population in
generation 29 of the fitness assay. Males and females were randomly paired, and were
transferred to a culture vial for oviposition. After 24 hours, the mated pairs were
discarded, and embryos were allowed to develop. Virgin adults from these vials were
collected, and the sexes were kept separated for 2 days. Then, individuals from each sex
were systematically paired to create a monogamous full-sibling mating treatment and a
monogamous outbred mating treatment. Each pair was kept in a culture vial with fresh
yeast for 24 hours, then transferred to a fresh vial without yeast and allowed to oviposit
for 24 hours. Mated pairs were then discarded and embryos allowed developing for 14
days. Adults were counted on the 10th and the 14th days after oviposition.
Statistical Analysis
Fitness assay. Regression analysis was used to measure the adaptation of treated
populations. Dependent and the independent variables were the number of successfully
developed adult individuals and generation, respectively. The test for the homogeneityof-regression was used to assess statistical significance of divergence between
19
treatments. Dependent variable, independent variable, and covariate were the number of
red-eyed individuals, treatment, and generation, respectively. Univariate analysis of
covariance (ANCOVA) was used to assess the statistical difference in vial density
between treatments. Dependent variable was the total number of adults per vial including
both experimental (red-eyed) and competitor (white-eyed) populations eclosed during the
entire collection period (Days 9-20). Independent variable, and covariable were treatment
and generation, respectively.
Development rate assays. Student’s t-test was used. In Low-competition
development and high-competition development assays, the ratio of the number of adults
from each collection day to the total number of adult from entire collection period was
compared between treatments in order to measure the difference in development rate
between treatments.
Inbreeding assay. Student’s t-test was used. The ratio of the number of inbred
adults to the number of outbred adults within a treatment were compared between
treatments in order to measure the difference in inbreeding depression between
treatments.
Each treatment had three independent lines and consisted of a large number of
individuals (>500), thus a normal distribution of the data is expected. Statistics software
used for analysis is SPSS 17.0. Microsoft Office Excel 2007 was used for visualization of
the data.
20
Location
All experimental procedure was conducted in Sequoia Hall, Room 38, at the
California State University, Sacramento.
21
RESULTS
Fitness Assay
Difference in adaptation rate in fitness. Regression analysis showed significant
adaptation in promiscuous populations (p=0.010) but not in monogamous populations
(p=0.340) (Table 3a, Figure 2). The test for the homogeneity-of-regression showed there
was no significant interaction (divergence) between treatments over the course of the
experiment (p=0.109) (Table 4a, Figure 2).
Difference in adaptation rate in productivity. Regression analysis did not show
significant adaptation in either promiscuous (p=0.862) or monogamous (p=0.364)
populations (Table 3b, Figure 3). The test for the homogeneity-of-regression showed
there was no significant interaction between treatments over the course of the experiment
(p=0.503) (Table 4b, Figure 3).
Vial density verification. ANCOVA showed the vial density in promiscuous
populations were significantly greater than that of monogamous populations (p=0.011)
(Table 5 and Figure 4), and the test for the homogeneity-of-regression showed there was
no significant interaction between treatments over generation (p=0.177) (Table 4c,
Figure 4) indicating that the difference in density was uniform throughout the
experiment.
22
Table 3. Regression analysis for fitness and productivity with respect to the first 16
generations of experiment.
Dependent
Variable
a) Fitness
Populations
Promiscuous
Monogamous
b) Productivity Promiscuous
Monogamous
Model
Generation
Generation
Generation
Generation
Unstandardized
Coefficient
(Slope)
15.730
4.173
1.388
-8.361
Standard
Error
5.737
4.313
7.909
9.079
t
2.742
0.967
0.175
-0.921
Sig.
0.010
0.340
0.862
0.364
23
Table 4. Test for the homogeneity-of-regression for fitness, productivity, and vial
density.
Dependent
Variable
a) Fitness
Type III Sum
Source
of Square
d.f. Mean Square
Treatment
4039.514
1
4039.514
Generation
151250.244
1 151250.244
Treatment *Generation
51924.699
1
51924.699
Error
1342065.446 68
19736.257
b) Productivity Treatment
287924.934
1 287924.934
Generation
7273.222
1
7273.222
Treatment *Generation
19458.969
1
19458.969
Error
2913248.337 68
42841.887
c) Vial Density Treatment
0.262
1
0.262
Generation
1258.717
1
1258.717
Treatment *Generation
1114.666
1
1114.668
Error
47210.956 68
598.547
F
0.205
7.664
2.631
Sig.
0.652
0.007
0.109
6.721 0.012
0.169 0.682
0.454 0.503
<0.001 0.983
2.103 0.152
1.862 0.177
Number of red-eyed individuals per
population (from the first two days of
collection period)
24
1200
y = 14.667x + 485.3
R² = 0.21354
1000
800
600
400
y = 4.1989x + 438.83
R² = 0.0375
200
0
0
Promiscuous
2
4
Monogamous
6
8
10
Generation
Linear (Promiscuous)
12
14
16
18
Linear (Monogamous)
Figure 2. Fitness of promiscuous and monogamous populations. Linear regression of
the number of adults from experimental populations (red-eyed) eclosed in Days 9-10 of
the main assay (Generations 1-16). Error bars indicate ± one standard error.
Number of red-eyed individuals per
population (from entire collection period)
25
2000
y = 1.7318x + 1208.8
R² = 0.00202
1600
1200
800
400
y = -3.1408x + 910.02
R² = 0.00843
0
0
Promiscuous
2
4
Monogamous
6
8
10
Generation
Linear (Promiscuous)
12
14
16
18
Linear (Monogamous)
Figure 3. Productivity of promiscuous and monogamous populations. Linear regression
of the number of adults from experimental populations (red-eyed) eclosed during the
entire collection period (Days 9-20) of the main assay. Error bars indicate ± one
standard error.
26
Table 5. ANCOVA of treatment and generation with respect to vial density.
Dependent
Variable
Vial Density
Source
Generation
Treatment
Error
Type III Sum of
Square
1258.717
4136.406
41815.833
d.f. Mean Square
1
4039.514
1
151250.244
69
51924.699
F
2.077
6.825
Sig.
0.154
0.011
Number of red-eyed and white-eyed
individuals combined per vial (from entire
collection period)
27
250
y = -0.6441x + 182.9
R² = 0.01741
200
150
100
y = -1.6965x + 174.54
R² = 0.15141
50
0
0
2
4
6
8
10
12
14
16
18
Generation
Promiscuous
Monogamous
Linear (Promiscuous)
Linear (Monogamous)
Figure 4. Vial density. Linear regression of the sum number of adults from
experimental (red-eyed) and competitor (white-eyed) populations eclosed in entire
collection period (Days 9-20) of the main assay (Generations 1-16). Error bars indicate
± one standard error.
28
Development Rate Assays
Low-competition development assay. Student’s t-test was conducted to compare
the development rate of population between treatments (Table 6, Figure 5). The variables
were the ratio of the number of adults eclosed in each collection day to the total number
of eclosed adults. There was no significant difference in the rate of eclosion between
treatments.
High-competition development assay. Student’s t-test was conducted to compare
the development rate between treatments (Table 7, Figure 6). The variables were the ratio
of the number of adults eclosed in each collection day to the total number of eclosed
adults. On the first day, there were more individuals eclosed in the promiscuous
treatment populations (p=0.014), whereas more monogamous individuals were eclosd on
the second day (p=0.020). By the second day of eclosion, 96.0% and 93.6% of total adult
were eclosed in promiscuous and monogamous populations respectively, and there was
no significant difference in the rate of eclosion between treatments when these 2 days
were combined (p=0.107).
Inbreeding Assay
Student’s t-test was conducted to compare the inbreeding depression between
treatments (Table 8, Figure 7). The variable was the ratio of the number of adults from
29
inbred matings to that from outbred matings in each treatment. There was no difference
in inbreeding depression between treatments (p=0.563).
30
Table 6. Student’s t-test for low-competition development assay.
Eclosion
Day
1
2
3
4
5
1+2*
Population
Mean
Variance
n
Promiscuous
0.388
4.65E-03
3
Monogamous
0.309
2.07E-03
3
Promiscuous
0.602
5.64E-03
3
Monogamous
0.687
2.32E-03
3
Promiscuous 7.16E-03
<0.001
3
Monogamous
1.86E-03
<0.001
3
Promiscuous 1.75E-03
<0.001
3
0
0
3
Promiscuous 1.75E-03
<0.001
3
Monogamous
1.90E-03
<0.001
3
Promiscuous
0.989
<0.001
3
4
Monogamous
0.996
<0.001
3
4
Monogamous
* Data from eclosion day 1 and 2 combined.
P-value
(one-tail)
d.f.
t
4
1.657
0.086
4
-1.657
0.086
4
2.123
0.051
4
1.000
0.187
4
-0.062
0.477
-1.256
0.138
31
Fraction matured
1
0.5
0
1
2
3
4
5
Emergence time (day)
Promiscuous
Monogamous
Figure 5. Rate of development with low competition. Error bars indicate ± one standard
error.
32
Table 7. Student’s t-test for high-competition development assay.
Eclosion
Day
1
2
3
4
5
1+2*
Population
Mean
Variance
n
Promiscuous
0.816
5.29E-03
3
Monogamous
0.607
2.52E-03
3
Promiscuous
0.143
3.87E-03
3
Monogamous
0.328
3.70E-03
3
Promiscuous
0.023
<0.001
3
Monogamous
0.046
<0.001
3
Promiscuous
0.011
<0.001
3
Monogamous
0.015
<0.001
3
Promiscuous 6.64E-03
<0.001
3
Monogamous
2.91E-03
<0.001
3
Promiscuous
0.960
<0.001
3
4
Monogamous
0.936
<0.001
3
4
*Data from eclosion day 1 and 2 combined.
P-value
(one-tail)
d.f.
t
4
4.121
0.007
4
-3.694
0.010
4
-2.503
0.033
4
-0.524
0.314
4
1.126
0.161
2.069
0.053
33
Fraction matured
1
0.5
0
1
2
3
4
Emergence time (day)
Promiscuous
Monogamous
Figure 6. Rate of development with high competition. Error bars indicate ± one
standard error.
5
34
Table 8. Student’s t-test for inbreeding assay.
Variables
Promiscuous
Inbred
Promiscuous
Outbred
Monogamous
Inbred
Monogamous
Outbred
Promiscuous
(inbred)/(outbred)
Monogamous
(inbred)/(outbred)
Mean
Variance
n
589.667
3.44E+03
3
779.333
1.11E+03
3
592.000
1.32E+03
3
755.333
1.28E+03
3
0.740
0.0102
3
0.780
0.0021
3
P-value P-value
(one-tail) (two-tail)
d.f.
t
4
-5.383
0.003
0.006
4
-1.756
0.076
0.153
4
-0.630
0.281
0.563
35
Inbreeding depression index
1
0.8
0.6
0.4
0.2
0
Promiscuous
Monogamous
Figure 7. Degree of inbreeding depression. Error bars indicate ± one standard error.
36
DISCUSSION
My experiment investigates whether sexual selection facilitates adaptation to a
competitive environment. The test for the homogeneity-of-regression (Table 4a,b) for the
fitness assay, which compares slopes of fitness functions and productivity between
promiscuously and monogamously mated populations, indicates that the adaptation rate
of these populations did not diverge after 16 generations of mating treatment, although I
observed a non-significant trend of higher adaptation rate of promiscuous population in
fitness (Figure 2).
Vial production was measured throughout the experiment to verify equal density
between treatments. The ANCOVA (Table 5) indicates that the population density of
promiscuous populations during the development period (from day 5 to day 10 in the
fitness assay; see table 2) was significantly higher than that of monogamous populations.
It suggests that the standardization of egg density in the fitness assay was not done
properly. Since it is well known that density can greatly affect the development rate of
individuals and the evolutionary course of populations (Mueller and Alaya 1981; Mueller
1988; Prasad et al. 2001), the results from the fitness assay can not be conclusive. In
other words, the result from the fitness assay can be due to the difference in population
density instead of the difference in the presence or absence of sexual selection.
No difference was seen in the development rate of promiscuous and monogamous
populations with a low degree competition after 30th generations (Table 6), however
37
promiscuous populations developed faster under high degree competition (Table 7).
Promiscuous populations were inadvertently propagated at higher density throughout the
experiment, and higher density selects for faster development rate. Therefore it is not
surprising that promiscuous populations developed faster at higher density where the
degree of competition is also high.
No difference in inbreeding depression was found between treatments (Table 8).
A previous study by Wigby and Chapman (2004) expressed concern that theory indicates
a similar experimental design causes a difference in inbreeding between treatments that
could affect the result of experiment. This concern has been addressed by evaluating the
prediction of differential inbreeding depression.
Various reasons can cause the difference in population density. In Drosophila
melanogaster, it is well known that interaction with males can greatly influence
physiology and behavior of females (Wolfner 1997). For example, a mated female
becomes less attractive to males (Cook and Cook 1975), decreases her receptivity to
further mating (Connoly and Cook 1973), and greatly increases her egg-laying rate
(Harndon and Wolfner 1995). Although all physical environmental factors were
controlled between promiscuous and monogamous populations, the difference in mating
treatment may have caused the difference in population density during the larval
development. In my experiment, I standardized the population density by reducing the
number of embryos laid on the surface of food medium in the oviposition vials. However
38
there may have been larvae that had already hatched from embryos. Because the first
instar larvae are translucent, they are harder to see. Now, if females in promiscuous
populations had laid more eggs due to the greater interaction with males, then there
would have been systematically more eggs in the oviposition vials of promiscuous
populations, hence creating the higher population density in promiscuous populations. If
repeated, the egg-density need to be more strictly controlled between populations.
39
CONCLUSION
This study investigated whether sexual selection facilitates populations in
adaptation to a competitive environment as predicted by the good genes hypothesis. The
rate of adaptation between promiscuous and monogamous populations to a competitive
environment was compared. The results indicate that both promiscuous and monogamous
populations did not measurably adapted to the given competitive environment. There was
no significant difference in the fitness between promiscuous and monogamous
populations. While the promiscuous populations had higher measured fitness due
apparently greater development rate, this result is inconclusive due to the systematic
difference in density over the course of the experiment. Repetition of this research
would require stricter control on population density.
40
LITERATURE CITED
Andersson, M. (1994) Sexual Selection. Princeton University Press.
Ashburner, M., Golic, K. G., and Hawley, R. S. (2005) Drosophila: a laboratory
handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Bateman, A. J. (1948) Intra-sexual selection in Drosophila. Heredity 2: 349-368.
Bettencourt, B. R., Kim, I, Hoffman, A. A., and Feder, M. E. (2002) Response to natural
and laboratory selection at the Drosophila hsp70 genes. Evolution 59: 1796-1801.
Cameron, E., Day, T., and Rowe, E. (2003) Sexual conflict and indirect benefits. Journal
of Evolutionary Biology 16: 1055-1060.
Chapman, T., Arnqvist, G., Bangham, J., and Rowe, L. (2003) Sexual conflict. Trends in
Ecology and Evolution 18: 41-47.
Connoly, K. and Cook, R. (1973) Rejection responses by female Drosophila
melanogaster: their ontogeny, causality, and effects upon the behavior of the
courting male. Behaviour 44: 142-166.
Cook, R. and Cook, A. (1975) Atractiveness to males of female Drosophila
melanogaster: effects of mating, age, and diet. Animal Behaviour 23: 521-526
Darwin, C (1871) The Descent of Man, and Selection in Relation to Sex. Murray,
London.
Fisher, R. A. (1915) The evolution of sexual preference. Eugenics Review 7: 184-192.
Fisher, R. A. (1930) The Genetic Theory of Natural Selection. Clarendon Press, Oxford.
Futuyma, D. J. (2005) Evolution. Sinauer Associates. Sunderland, MA.
Gowaty, P. A., Kim, Y., Rawlings, J. and Anderson A. A. (2010) Polyandry increases
offspring viability and mother productivity but does not decrease mother survival
in Drosophila pseudoobscura. Proceedings of the National Academy of Sciences
of the United States of America 107: 13771-13776.
41
Harndon, L. A., and Wolfner, M. F. (1995) A Drosophila seminal fluid protein,
Acp26Aa, stimulates egg-laying in females for one day following mating.
Proceedings of the National Academy of Sciences of the United States of America
92: 10114-10118.
Holland, B. (2002) Sexual selection fails to promote adaptation to a new environment.
Evolution 56: 721-730.
Iwasa, Y., and Pomiankowski, A. (1999) Good parent and good genes models of
handicap evolution. Journal of theoretical Biology 200: 97-109.
Jones, A. G. and Ratterman, N. L. (2009) Mate choice and sexual selection: what have
we learned since Darwin? Proceedings of the National Academy of Sciences of
the United States of America 106: 10001-10008.
Kirkpatrick, M. and Ryan, M. J. (1991) The evolution of mating preferences and the
paradox of lek. Nature 350: 33-38.
Kirkpatrick, M. (1996) Good genes and direct selection in the evolution of mating
preferences. Evolution 50: 2125-2140.
Kokko, H., Brooks, R., Jennions, M. D., and Morley, J. (2003) The evolution of mate
choice and mating biases. Proceedings of the Royal Society of London B 270:
653-664.
Kokko, H., Jennions, M. D., and Brooks, R. (2006) Unifying and testing models of
sexual selection. Annual Review of Ecology Evolution and Systematics 37: 43-66.
Kotiaho, J. S., and Puurtinen, M. (2007) Mate choice for indirect genetic benefits:
security of the current paradigm. Functional Ecology 21: 638-644.
Mead, L. S. and Arnold, S. J. (2004) Quantitative genetic models of sexual selection.
Trends in Ecology and Evolution 19: 264-271.
Møller, A. P. and Alatalo, R.V. (1999) Good-genes effects in sexual selection.
Proceedings of the Royal Society of London B 266: 85-91.
Moore, A. J., Gowaty, P. A., and Moore, P. J. (2003) Female avoid manipulative males
and live longer. Journal of Evolutionary Biology 18: 523-530.
42
Mueller, L. D. and Ayala, F. J. (1981) Trade-off between r-selection and K-selection in
Drosophila populations. Proceedings of the National Academy of Sciences of the
United States of America 78: 1303-1305.
Mueller, L. D. (1988) Evolution of competitive ability in Drosophila by densitydependent natural selection. Proceedings of the National Academy of
Sciences of the United States of America 85: 4383-4386.
Orians, G. H. (1961) The ecology of blackbird (Agelaius) social systems. Ecological
Monographs 31: 285-312.
Paker, G. A. (1979) Sexual selection and sexual conflict. Pp. 123-166. in M. S. Blum and
N. A. Blum, eds. Sexual selection and reproductive competition in insects.
London: Academic Press.
Parker, G. A. (2006) Sexual conflict over mating and fertilization: an overview.
Philosophical Transactions of the Royal Society B 361: 235-259
Partridge , L. (1980) Mate choice increases a component of offspring fitness in fruit flies.
Nature 283: 290-291.
Prasad, N. G., Shakarad, M., Anitha, D., Rajamani, M., and Joshi, A. (2001) Correlated
response to selection for faster development and early reproduction in
Drosophila: the evolution of larval traits. Evolution 55: 1363-1372.
Promislow, D. E. L., Smith, E. A., Pearse, L. (1998) Adult fitness consequences of
sexual selection in Drosophila melanogaster. Proceedings of the National
Academy of Sciences of the United States of America 95: 10687-10692.
Pruutinen, M., Ketola, T, and Kotiaho, J. S. (2009) The good-genes and compatiblegenes benefits of mate choice. American Naturalist 175: 741-752.
Schaeffer, S. W., Brown, C. J., and Anderson, W. W. (1984) Does mate choice affect
fitness? Genetics 107: S94
Stewart, A. D., Morrow, E. H., and Rice, W. R. (2005) Assessing putative interlocus
sexual conflict in Drosophila melanogaster using experiment evolution.
Proceedings of the Royal Society of London B 272: 2029-2035.
43
Trivers, R. L. (1972) Parental investment and sexual selection. Pp. 136-179 in B.
Campbell, ed. Sexual selection and the decent of man: 1871-1971. Heinemann,
London.
Weatherhead, P. J., and Robertson, R. J. (1979) Offspring quality and the polygyny
threshold: “the sexy son hypothesis.” American Naturalist 113: 201-208.
Welch, A. M., Semlitsch, R. D., Gerherdt, H. C. (1993) Call duration as an indicator of
genetic quality in male gray tree frogs. Science 280: 1928-1930.
Wigby, S. and Chapman, T. (2004) Female resistance to male harm evolves in response
to manipulation of sexual conflict. Evolution 58: 1028-1037.
Williams, G. C. (1966) Adaptation and natural Selection: A Critique of Some Current
Evolutionary Thought. Princeton University Press, Princeton, N.J.
Wolfner, M. F. (1997) Tokens of love: functions and regulation of Drosophila male
accessory gland products. Insect Biochemistry and Molecular Biology 27: 179192.
Zahavi, A. (1975) Mate selection - a selection for a handicap. Journal of Theoretical
Biology 53: 205-214.