Carbon in anaerobic aquatic environments

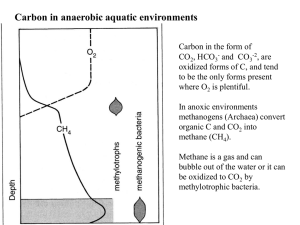
Carbon in anaerobic aquatic environments
Carbon in the form of
CO
2
, HCO
3
and CO
3
-2 , are oxidized forms of C, and tend to be the only forms present where O
2 is plentiful.
In anoxic environments methanogens (Archaea) convert organic C and CO
2 methane (CH
4
).
into
Methane is a gas and can bubble out of the water or it can be oxidized to CO
2 by methylotrophic bacteria.
Methanogens are not true bacteria, they belong to the Archaea
Most methanogens can grow on CO
2 and H
2 as their sole energy source:
Chemoautotrophs —chemical bond energy is their energy source they utilize CO
2 as their C source http://faculty.plattsburgh.edu/jose.deondarza/images/Organisms/methanogen.jpg
0
+4
C-transformations in aerobic and anaerobic environments
Oxidation state
-4
Where do we find methanogens?
•Under anaerobic conditions organic molecules break down to methane instead of CO
2
—This process is facilitated by methanogens (Archaea), which are chemoautotrophic bacteria.
•They utilize the energy released from 2H
2 biomass.
+ Organic C (CH
2
O)→CH
4
+ H
2
0 to build their
How to assign Oxidation numbers
We keep track of the e transfer using Oxidation numbers (Ox#)
For each e transferred the Ox# changes by 1
2H
2
+ O
0 0
2
2H
2
O
+1 -2
Some rules for Oxidation numbers
1. In free elements Ox# = 0
2. For ions with one atom Ox# = charge. eg H + Ox# of H + = 1
3. Ox# of O in most compounds is -2 ,
4. Ox# of H in most compounds is +1 ,
5. For a complex ion like SO
4
-2 , the net Ox# = charge (Thus S= +6 )
Chemical equation for the reduction of CO
2 by H
2
CO
2
H
2
CH
4
H
2
O
E(energy)
CO
2
4
H
2
CH
4
2
H
2
O
(
4) ( 0 ) (-4) (
1)
8
e
-
are accepted per
C
1
e
-
is donated per H
Nutrition and Metabolic Diversity
Four nutritional categories
Carbon Source
CO
2
Organic
Light
Photoautotroph
Photoheterotroph
Chemical
Chemoautotroph
Chemoheterotroph
Solar Saltern Plant: a typical habitat of Halobacterium salinarium
Functional classification?
•found in water saturated or nearly saturated with salt (Halophiles) and rich in organic matter.
•Large blooms appear reddish, from the pigment bacteriorhodopsin . This pigment is used to absorb light, which provides energy to create ATP .
•The process is unrelated to other forms of photosynthesis involving electron transport, however, and halobacteria are incapable of fixing carbon from carbon dioxide .
http://www.biochem.uni-luebeck.de/public/groups/metalloproteins/hubmacher/Hubmacher_Abb2.jpg
The Cycling of Nitrogen
N is an important nutrient that frequently limits primary productivity in aquatic ecosystems
It is rare in the earth’s crust, but makes up 79% of the atmosphere (N
2
)
(oxidation state =0)
Most algae and plants require
NO
3
¯(+5) (NO
2
¯ ) (+3) or NH
3 make proteins
(NH
4
+ ) (-3)to synthesize amino acids to
N-fixing microorganisms can take up N
2
N
2
+ 3H
2
→ 2NH
3 and convert it to NH
Many plants have N-fixing mutualists (eg Azolla )
3
Denitrifying bacteria can convert NO
3
¯ back to N
2
Azolla , an aquatic fern used in rice culture
•The leaves of this aquatic fern have cavities that harbour filamentous cyanobacteria
Anabaena azollae
•The large cells (heterocysts) are specialized for N-fixation
•Traditional rice farming in many countries involve planting Azolla to build up N concentrations in rice paddy.
0
+1
+3
The Nitrogen cycle involves many different oxidation states, and the redox processes are facilitated by plants and wide variety of bacteria
Chemoheterotrophs (CH)
-3
CH
PA
Nitrite
+5
CH
Photoautotrophs (PA) Chemoautotrophs(CA)
-2
0
+4
+6
Photoautotrophs (PA) Chemoautotrophs(CA)
Chemoheterotrophs (CH)
CH
PA
Streams draining mine tailings are extremely acidic—the effect of Thiobacillus oxidizing pyrites and iron.
Thiobacillus ferrooxidans oxidizes both the iron,
Fe(+2) to Fe(+3) and the sulphur in the pyrites,
S(-1) to S(+6) using molecular oxygen.
This reaction splits water to produce a great deal of acid.
How do you suggest that mine tailings should be stored?
2 FeS
2
7 .
5 O
2
4 H
2
O
4 SO
4
2
Fe
2
O
3
8 H
Energy
Desulfovibrio : Sulfate reducing bacteria commonly found in anaerobic aquatic environments with high levels of organic material, such as mud in lakes and ponds.
•have metal reductases which can precipitate metal sulfides from the water—
•bioremediation potentials for toxic radionuclides such as uranium by a reductive bioaccumulation process.
Sulfate reduction can absorb H+ and counteract acid rain
They also contribute to methylation of
Mercury
Chemical equation for the oxidation of acetate by sulfate
CH
3
COO
SO
4
2
H
S
H
2
O
CO
2
E
CH
3
COO
SO
4
2
H
S
2
H
2
O
2
CO
2
(0) (
6) (-2) (
4)
8
e
-
are accepted per
S
4
e
-
are donated per C
Where do sulphate reducing bacteria fit in the functional classification?