Carbon in the form of CO , HCO and CO
advertisement

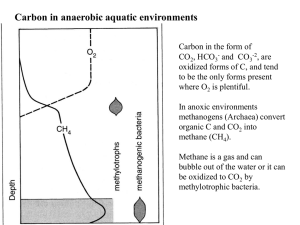
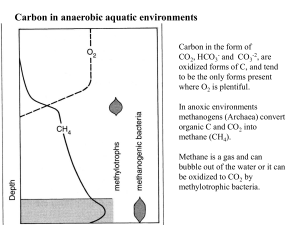
Carbon in the form of CO2, HCO3- and CO3-2, are oxidized forms of C, and tend to be the only forms present where O2 is plentiful. In anoxic environments methanogens (Archaea) convert organic C and CO2 into methane (CH4). Methane is a gas and can bubble out of the water or it can be oxidized to CO2 by methylotrophic bacteria. Methanogens are not true bacteria, they belong to the Archaea Most methanogens can grow on CO2 (or organic C)and H2 as their sole energy source: Chemoautotrophs —chemical bond energy is their energy source they utilize CO2 as their C source http://faculty.plattsburgh.edu/jose.deondarza/images/Organisms/methanogen.jpg C-transformations in aerobic and anaerobic environments Oxidation state Where do we find methanogens? -4 0 +4 •Under anaerobic conditions organic molecules break down to methane instead of CO2—This process is facilitated by methanogens (Archaea), which are chemoautotrophic bacteria. •They utilize the energy released from 2H2 + Organic C (CH2O)→CH4 + H20 to build their biomass. How to assign Oxidation numbers We keep track of the e- transfer using Oxidation numbers (Ox#) For each e- transferred the Ox# changes by 1 2H2 + O2 0 0 2H2O +1 -2 Some rules for Oxidation numbers 1. In free elements Ox# =0 2. For ions with one atom Ox# = charge. eg H+ Ox# of H+ = 1 3. Ox# of O in most compounds is -2, 4. Ox# of H in most compounds is +1, 5. For a complex ion like SO4-2 , the net Ox# = charge (Thus S=+6) Carbon and Oxygen play a major role in biological energy transformations Oxidation means giving up electrons, and reduction means taking on electrons (─) The oxidation state of C in CO2 is +4, in Carbohydrates is 0 How many electrons are taken up by each C during photosynthesis? Chemical equation for the reduction of CO2 by H2 CO2 H 2 CH 4 H 2O E(energy) CO 2 4 H 2 CH 4 2 H 2O ( 4) (0) (-4) - ( 1) 8 e are accepted per C - 1 e is donated per H Nutrition and Metabolic Diversity Four nutritional categories Carbon Source Energy Source CO2 Organic Light Photoautotroph Photoheterotroph Chemical Chemoautotroph Chemoheterotroph