The Cycling of Nitrogen aquatic ecosystems
advertisement

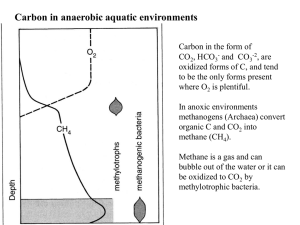
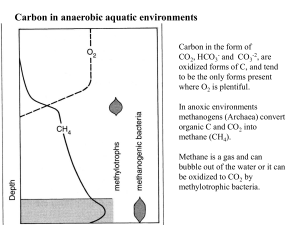
The Cycling of Nitrogen N is an important nutrient that frequently limits primary productivity in aquatic ecosystems It is rare in the earth’s crust, but makes up 79% of the atmosphere (N2) (oxidation state =0) Most algae and plants require NO3¯(+5) (NO2 ¯) (+3) or NH3 (NH4+) (-3)to synthesize amino acids to make proteins N-fixing microorganisms can take up N2 and convert it to NH3 N2 + 3H2 → 2NH3 Many plants have N-fixing mutualists (eg Azolla) Denitrifying bacteria can convert NO3¯ back to N2 Azolla, an aquatic fern used in rice culture •The leaves of this aquatic fern have cavities that harbour filamentous cyanobacteria Anabaena azollae •The large cells (heterocysts) are specialized for N-fixation •Traditional rice farming in many countries involve planting Azolla to build up N concentrations in rice paddy. •Nutrients like N and P tend to accumulate in the hypolimnion during summer stratification— sedimentation. •In eutrophic lakes the deep layers become very depleted in O2 •NO3-—the most oxidized form of N occurs highest in the water column where there is O2 present •NH4+ or NH3, the most reduced form is prevalent deep where O2 is absent or nearly so •N2O and NO2-—are intermediate oxidation states The Nitrogen cycle involves many different oxidation states, and the redox processes are facilitated by plants and wide variety of bacteria Chemoheterotrophs (CH) -3 CH PA 0 +1 +3 Nitrite CH +5 Photoautotrophs (PA) Chemoautotrophs(CA) This graph shows Nitrate concentrations In large rivers as a function of human population density This graph shows Nitrate export from large river watersheds as a function of human population density Question?? Explain Prairie rivers and watersheds are ‘high’ in Nitrates and Nitrate export, even though population density is low.