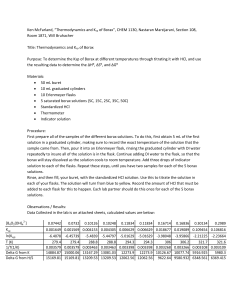
Borax in Nevada A study of composite minerals

Borax in Nevada
A study of composite minerals
Borax Begins in Nevada
Rich Moreno – Backyard Traveler
• A stiff wind sweeps across a powdery, alkali lake bed and blows through the tufts of sagebrush growing around a few stone walls and foundations. They are all that remain of the mining camp of Marietta.
Founded in the late 1870s, Marietta was not a typical central Nevada mining camp because its fortunes were not based on gold and silver, but on borax and salt.
Originally known as Teels Marsh, the area was first developed in 1867 when salt was mined in the flat and transported by camel train to Virginia City’s booming mines (salt was used in processing ore).
In 1872, Francis M. Smith, who would later become renowned as “Borax” Smith, was working the salt fields in nearby Columbus and spotted the borate potential of the marsh. He and his brother took samples from the dry lake bed, which proved to be rich in borax, and staked much of the area.
Full scale borax mining begin within months of the discovery as the Smith brothers and other miners began constructing borax plants at the southeastern end of the marsh. Records indicate that wagon trains hauled the material all the way to a train station at Wadsworth, about 115 miles north.
Borax in Nevada
• The Teels Marsh borax field was not only an important discovery for Nevada but allowed the Smiths to create the Teels Marsh Borax Company, a predecessor to the Pacific Borax Salt & Soda Co., which in the late 19th century would control the world borax market.
Smith's genius was that he recognized the value of borax if marketed correctly. Prior to his efforts, borax was mined in Europe and used primarily in pharmaceuticals.
Smith would eventually move west to larger borax (also known as colmanite) discoveries in Death Valley and become famed for his 40-mule teams that carried the ore out of the Death Valley area. Of course, he superbly marketed his mule trains so that they and Borax became household names.
The discovery of the richer borax deposits at Death Valley eventually signaled the end of Marietta. By the 1890s, the borax operations were abandoned and the town slid into oblivion.
The drive to Marietta allows visitors to view the real Nevada outback. The road winds through rolling sage-covered foothills before dropping down into the flat, treeless, alkali valley that is the location of
Teels Marsh and, at the southeast end of the marsh, the ruins of Marietta.
Marietta is located 56 miles southwest of Hawthorne via U.S. Highway 95, then west on State Route 360 to a marked and maintained dirt road
Nevada Counties
Fold a Crystal Shape
Borax makes a Tetragonal shaped crystal
Tetragonal
Amethyst Crystal
Minerals are Crystals
Rough
Diamond
Carbon
Quartz Crystals
Crystal Shapes
Crystal Formation
• A crystal is a solid material whose atoms, molecules and ions are arranged in an orderly repeating pattern extending in all three spatial dimensions.
• The scientific study of crystals and crystal formation is crystallography .
• The process of forming a crystalline structure from a fluid or from materials dissolved in the fluid is often referred to as crystallization .
• The crystal structure that the fluid will form depends on:
– the chemistry of the fluid, t
– the conditions under which it is being solidified,
– the ambient pressure .
Borax Crystal Snowflakes
• What To Do:
• Twist together three pipe cleaners in the center to make a 6pointed star.
• Tie a piece of string to one end of the star. Connect the string to the next point by twisting it around the pipe cleaner.
Continue around until you connect all the points together with the string, making a snowflake skeleton (see the picture).
• Tie another piece of string to one of the pipe cleaner points and tie the other end around the pencil. Place the snowflake in the jar with the pencil resting across the mouth of the jar.
Make sure that the snowflake hangs without touching any part of the jar. Take the snowflake out of the jar.
• Hang your snowflake in the jar so that it is completely covered in the solution. Let it sit overnight. Gently remove your now crystal-covered snowflake in the morning and let it dry by hanging it in a dry jar.