Light and Atoms Why study the behavior of light and atoms?
advertisement
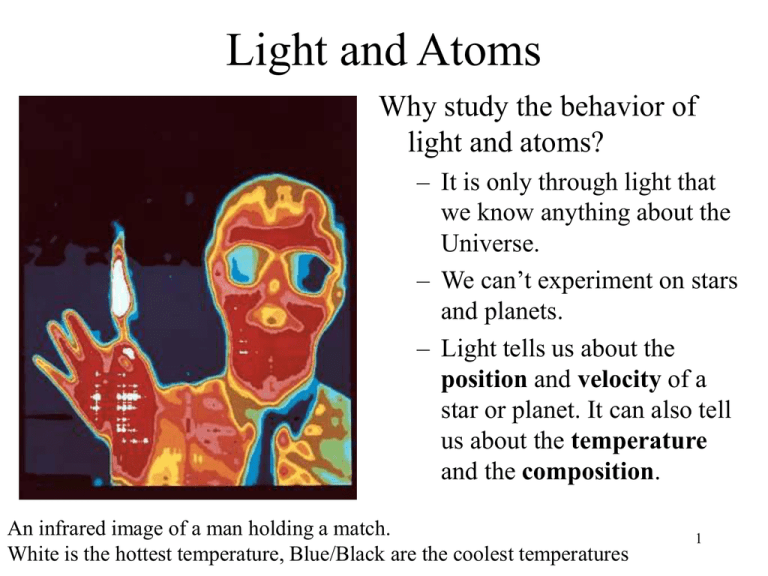
Light and Atoms Why study the behavior of light and atoms? – It is only through light that we know anything about the Universe. – We can’t experiment on stars and planets. – Light tells us about the position and velocity of a star or planet. It can also tell us about the temperature and the composition. An infrared image of a man holding a match. White is the hottest temperature, Blue/Black are the coolest temperatures 1 Light & Atoms • Atoms interact with light by absorbing, emitting and bending light • Atoms leave their unique signature or “fingerprint” in the light they emit or absorb – this can tell us what stars and planets are made of – astronomers see these “fingerprints” in light from objects in distant galaxies. • Atoms in our own atmosphere can blur and absorb light from distant objects 2 The Nature of Light Light is an electromagnetic wave • The speed of light (c) is constant in a vacuum • Light has particle-like properties (photons) and wave-like properties (wavelength and frequency) • Which way you describe light depends on the kind of observation you are making. 3 The Nature of Light • Light has a wavelength and a frequency – wavelength () - distance between wave crests – frequency () - number of wave crests that pass a point in 1 second • When you tune your radio you are actually changing the frequency. • A piano will emit different frequencies of sound based on which key you strike. 4 Wavelength and Frequency The wavelength () and the frequency () are related to the speed (c) by the formula =c If the wavelength increases the frequency must decrease for the speed to stay the same 5 Color and Frequency • The frequency and color of light are related. Red light for example has a lower frequency than blue light. • White light is a mixture of light of many different frequencies. A prism can break light into a rainbow (spectrum) of 6 colors. Wave vs. Photon • Wavelength/Frequency – Short wavelength: blue – Long wavelength: red • Photon energy – High energy – Low energy • Wave Amplitude – Small amplitude – Large amplitude • Photon flux (num. photons/area) – Low photon flux – High photon flux 7 Electromagnetic Radiation • Visible light is just one form of electromagnetic radiation. • Together they form the electromagnetic spectrum. They differ in their wavelength, frequency and energy. • However specifying the wavelength, frequency or energy uniquely characterizes the form of electromagnetic radiation. 8 The Energy of EM Radiation The energy (E) carried by electromagnetic 10 – 10 K radiation is related 10 – 10 K to the frequency (). 1,000 – 10,000 K X-rays have a higher 10 – 1,000 K frequency and T < 10 K higher energy than radio waves for example. T > 108 K 6 8 4 6 9 The Energy of EM Radiation The energy (E) carried by electromagnetic Hot radiation is related massive stars to the frequency (). Planets/Stars/Galaxies X-rays have a higher Cool Stars / Warm dust frequency and Planets Cold gas / Accelerated higher energy than electrons / Cosmic Background Radiation radio waves for example. Pulsars / Black Hole Accretion Supernovae / Hot tenuous gas 10 Multi-Wavelength Astronomy • Different physical processes produce light at different wavelengths. • To better understand a physical system (planet, star, galaxy), observe it in as many energy (wavelength) bands as possible. – Choose the bands to match the energy range for the physical process of interest. 11 Multi-Wavelength Astronomy • • • • • The Sun Supernova Remnant Cas A Supernova Remnant M1, The Crab Nebula Giant Elliptical Galaxy M87 Peculiar, Interacting Galaxy Centaurus A 12 Wien’s Law • Wien’s law allows astronomers to determine the temperature of a star. • The wavelength at which a star is brightest is related to its temperature • Hotter objects radiate more strongly at shorter wavelengths • Blue has a shorter •Objects can emit radiation at many different wavelengths. wavelength than red, so •The wavelength at which a star is brightest hotter objects look bluer. is related to its temperature. •This is Wien’s Law 13 When can you use Wien’s Law? • • • • Only for objects that emit light not for those that reflect light Light emitted by hot, solid objects obey Wien’s Law Can not use with gases unless they are of a high density The Sun and other stars obey Wien’s Law since the gases they are composed of remain at a high density (at least up to the outermost layers of the star). 14



