Thermodynamics the study of energy transformations and transfer
advertisement
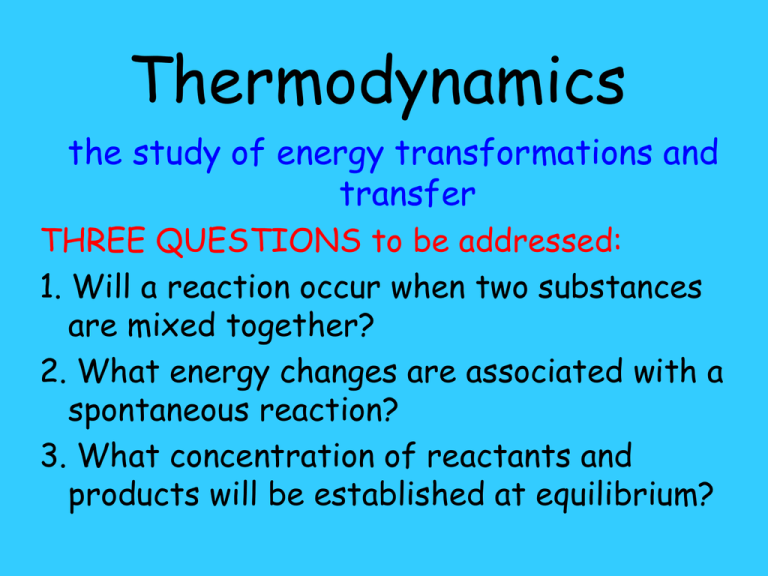
Thermodynamics the study of energy transformations and transfer THREE QUESTIONS to be addressed: 1. Will a reaction occur when two substances are mixed together? 2. What energy changes are associated with a spontaneous reaction? 3. What concentration of reactants and products will be established at equilibrium? surroundings system energy transfer energy transformations In chemistry the system will be a chemical or physical change. Laws of Thermodynamics • 1st - the total amount of energy in the universe is constant (energy is conserved) • 2nd - in any spontaneous change the entropy of the universe increases • 3rd - the entropy of any pure, perfect crystalline solid at absolute zero is equal to zero Thermodynamic quantities • Entropy • Enthalpy • Free Energy • Relate to the Equilibrium Constant











