Tracheobronchomalacia The ugly, but treatable step-cousin of the COPD family

Tracheobronchomalacia
The ugly, but treatable step-cousin of the COPD family
Colin McKnight, MSIII, 2007
Presentation Outline
• Brief review of “COPD” definition, epidemiology and economic costs
• Tracheobronchomalacia
– Definition
– Pathophysiology
– Known epidemiology and natural history
– Evolving methods of diagnosis
– New treatment strategies and outcomes
– Significance
– Proposed study design
COPD: An unpopular disease from the start
An Interesting perspective from
1976:
• “Chonic bronchitis with it accompanying emphysema is a disease on which a good deal of wholly unmerited sympathy is frequently wasted. It is a disease of the gluttonous, bibulous, otiose, and obese and represents a well-deserved nemesis for these unlovely indulgences . . .
The majority of cases are undoubtedly due to surfeit and selfindulgence”
Fletcher C, Peto R., Tinker C, Speizer FE. The natural history of chronic bronchitis and emphysema. New York: Oxford University Press, 1976.
Risk Factors for “COPD”
• “Well established”:
– Tobacco smoke
– α1-Antitrypsin deficiency
– Environmental exposures
• “Good evidence”:
– Air pollution
– Low birth weight
– Childhood respiratory infection
– Atopy
– Low socioeconomic status
– Alcohol intake
*Viegi
How Far Have We Really Come
Since
1976
?
• Research of ‘tobacco induced’ cancers such as lung cancer, receive only 10-15% of that for breast or prostate cancer when dollars spent per death are examined*
• Perhaps our willingness to blame the health of our patients on smoking has presented us from a more sophisticated understanding of obstructive pulmonary physiology.
*Dennis
Which Diseases Lead to Obstructive
Pulmonary Physiology?
• A conglomeration of various pathophysiological processes, of which the treatment varies substantially:
– Emphysema
– Chronic Bronchitis
– Asthma
– Bronchiectasis
– Bronchiolitis obliterans
– Tracheobronchomalacia
Assumptions
• Unfortunately, patients with obstructive pulmonary physiology are often assumed to have emphysema
Picture
• This is particularly true for those who smoke cigarettes
GOLD criteria for COPD diagnosis
• GOLD* criteria:
– airflow limitation
– not fully reversible.
– usually both progressive
– Usually associated with an abnormal lung inflammatory response to noxious particles or gases
Current GOLD definition of airflow limitation is FEV1:FVC ratio <70%
Stage I:
Stage IIA:
Stage IIB:
Stage III:
FEV
1
> 80%
FEV
1
50-80%
FEV
1
30-50%
FEV
1
< 30%
*
GOLD: Global initiative for
Chronic Lung Disease
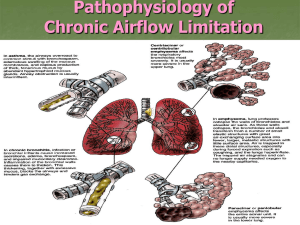
“COPD”
• Emphysema
• Sx:
– Severe, constant dyspnea, mild cough
• Chronic Bronchitis
• Sx :
– Intermittent dyspnea
– Copious sputum
• PE:
– Pink Puffers:
•
Tachypneic
• Non-cyanotic
• Thin
• Diminished Breath sounds
• PE :
– Blue Bloaters:
• Cyanotic
• Obese
• Edematous
• Rhonchi and Wheezes
COPD “diagnosis”
• Individuals with irreversible obstructive pulmonary physiology and the following non-specific findings are unfortunately often lumped together under one
“diagnosis”:
– Dyspnea
– Cough
– Sputum
COPD Epidemiology
• 2000 estimate was >10 million diagnosed in U.S.
• Millions more are undiagnosed.
• COPD is the fourth leading cause of chronic morbidity and mortality in the
US and within a decade will surpass stroke to become the third leading cause of death
Current COPD treatments
– C orticosteroids
– O xygen
– P revention
• Smoking cessation
• vaccination
– D ilators
• Anticholinergics
• B2-agonists
• Theophyllline
– Rehabilitation
• Current goals of treatment:
– Slow further progression
– Reduce exacerbation hospitalizations
– Maximize activity
Only Oxygen has been shown to improve mortality
A Tremendous Burden
• The economic impact of
COPD in the US:
– 1993: $23.9 Billion*
• Direct costs $14.7 billion
• Indirect costs $9.2 billion
• 2002: $32.1 billion *
*Sullivan
*National Heart and Lung Institute
• Annual mean individual cost is $5,646*
– $2,000 for mild cases
– $16,000 for severe cases
• This Probably underestimates true burden because COPD often not:
– “primary reason for hospital visit”
– “cause of death”
*Wouters
*Halpan
My Frustrations
• COPD expenditures represent a tremendous cost to a health care system that is already on the verge of collapse and does not provide basic services to a large proportion of the population.
• Treatments offer very little hope of improvement.
• Despite expensive new medications, relative gains are little.
Tracheomalacia and
Tracheobronchial malacia
• Definition: Malacia = Weakness
• Tracheomalacia (TM) weakness of the trachea
• Tracheobronchomalacia (TBM) weakness of the trachea and main stem bronchi
*This presentatin will focus on adult, acquired tracheobronchomalacia rather than the congenital form of the disease
Postulated Pathophysiology
• Flaccid posterior membranous wall of the trachea bows anteriorly during dynamic expiration in response to elevated intrathoracic, extratracheal pressure.
• This is a dynamic process that is accentuated by forced expiration, thus routine AP and Lateral chest radiographs often show no abnormality.
Inspiration:
Decreased extratracheal pressure
Expiration:
Increased extratracheal pressure
Trachea cross section
During inspiration, the trachea dilates and lengthens.
Normal trachea Tracheomalacia
A weakened posterior tracheal wall leads to an exaggeration of the normal narrowing during expiration.
Etiology
• Excessive bowing is thought to be due to reduction/atrophy of the longitudinal elastic fibers of the pars membranacea or impaired cartilage integrity.
• Presumed causes of acquired TM/TBM
– Treacheostomy
– Intubation
– Tobacco smoke
– Radiation/previus surgery
– Recurrent infections
(with or without cigarette smoking)
Symptoms of TM and TBM
• Dyspnea
• Cough
• Sputum Production
• May have:
– Inspiratory wheezing/stridor
– “Barking seal cough”
– Multiple admissions for
“asthma exacerbations” with only modest treatment results
• These are non-specific , and very often attributed to:
– Emphysema
– Chronic Bronchitis
– Cigarette Smoking
– Asthma
• This is an obscure, insignificant disease…
Right???
NO!!!
Population studies indicate very significant prevalence within certain populations:
-Jolinen et al reported finding TM in 50 of
214 patients (23%) with chronic bronchitis who were examined bronchoscopically
-Palombini revealed that
TBM may be present in 10-15% of patients who see a pulmonologist for chronic cough
Malacia epidemiology continued…
• The most recent epidemiological data comes from a Japanese study that demonstrated TBM was present 542 of 4,283 patients (12.7%) with pulmonary disease who underwent bronchoscopy. (72% of this study were between
50-80years old).
• It is generally accepted that TBM is seldom found in individuals without some evidence of obstructive lung disease
* Ikeda
A Progressive disease
• TM and TBM have been shown in various studies to be progressive diseases. In one longitudinal study, of 94 patients with TM and
TBM with an average follow-up period 5.2 years progression had occurred in 6 of 9 patients.
Zero patients showed improvement of their malacia.
• It has been postulated that TM causes a defect in secretion clearing, allowing for pathology to spread down the bronchial tree.
A relatively good candidate for
“COPD” intervention
• Thus, TM represents a progressive pathophysiologic process where it may be possible to avoid further progression.
• Unlike emphysema, chronic bronchitis, and bronchiolitis obliterans, the defect is discrete and localized.
• Thus, this disease offers a rare target for mechanical intervention of “COPD”.
3 Further Questions
• Can this TBM be diagnosed in reliable, non-invasive manner?
• Are there effective interventions available?
• Are these interventions cost effective?
Diagnosis
• Bronchoscopy is the established gold standard
• Generally accepted that 50% loss of tracheal lumen during expiration is diagnostic of TM
A
B
A: Cross section in a healthy control subject during inhalation (left), exhalation (middle) and forced exhalation. SIs change from .95 to 0.93 to .87.
B: Tracings of a TBM patient showing bowing of the posterior membrane. SIs change from .95 to .65 to .14.
*Loring
A
A Non-Invasive Method of
Diagnosis
• Gilkeson showed in 2000 that the degree of TBM defined by bronchoscopy correlates very well with dynamic CT findings.
Image A: axial CT showing trachea of normal caliber
Image B: dynamic CT showing crescentric bowing of posterior membranous trachea
B
Evolution of Diagnosis
• In 2003 Zhang et al showed that Dynamic
CT was highly sensitive at detecting
TBM.
• N=20. 10/10 TBM patients were identified by CT. 0/10 control subjects were falsely labeled with malacia.
• Additionally, Zhang showed that a low dose dynamic CT was statistically equal at detecting TBM, thus indicating that high dose CT is not necessary for TBM diagnosis.
Demonstration of Air Trapping
Left: End inspiratory CT
Right: Dynamic expiratory CT at a similar level on the same patient. White arrows indicate areas of air trapping in this TBM patient.
Zhang et al demonstrated that patients with TBM defined by dynamic expiratory
CT have statistically more severe air trapping. This air trapping has been postulated to be worsened in TBM patients from difficulties clearing secretions.
Secretions are though to accumulate, thus inducing chronic inflammation of small airways, leading to air trapping.
*Zhang, 2003
Use of PFTs in Diagnosis?
-The degree of obstruction indicated by FEV1 does not correlate well with presence of TBM.
-In TBM patients there is often a rapid decline in the maximal expiratory flow after a sharp peak associated with the collapse of central airways due to negative transmural pressure.
PFTs therefore can be useful in raising suspicion of TBM, but they are not diagnostic.
Flow volume loop in
TM patient.
*Carden
Stenting
• Metal stents can be placed by bronchoscopy. They can expand dynamically and preserve mucociliary function, though breakage has caused severe complications, including death.
• Silicone stents can be easily repositioned and removed.
• Trials including stented patients have shown that stents lead to subjective and objective improvement in respiratory symptoms.
• Further larger trials are needed.
Tracheoplasty
• Current methods call for surgical reinforcement of the posterior membranous wall with tracheoplasty enhancing the rigidity of the structure making it less susceptible to bowing during expiration.
Benefits of Tracheoplasty
Wright et al, 2005
• n=14
• Significantly increased
– mean forced expiratory volume (p=.009)
– peak flow rate (p=.00001)
• All patients had decreased
– Dyspnea
– Cough
– secretion retention and
• All reported increased activity
Baroni RH, 2004
• N=5
• All showed a normal shaped trachea during inspiration following treatment
• 4/5 showed normal shape on expiration
• All patients reported subjective improvement in symptoms
•
0/5 experience significant complications
C
A
Dynamic CT Demonstration of
Improvement Following Tracheoplasty
A: Preoperative end inspiratory
B: Preoperative dynamic expiratory
B
C: Postoperative end-inspiratory
D: Postoperative dynamic expiratory
D
*Baroni
PFT Change Following Tracheoplasty
Preoperative 2 Months Postoperative
Flow volume curves in a 57-year-old man with 7 years of progressive dyspnea, wheezing, cough, and respiratory infections.
*Wright
Further Benefits of TBM Diagnosis and Treatment
• Best evidence indicates depression is present in 25-50% of COPD patients.
• 28% of COPD patients say they are embarrassed about the disease, and the same number say they feel defeated (particularly women and younger patients).
• An overwhelming portion of patients believe they brought on the disease because of a smoking habit. guilt, stigma, reinforcement
• Patients with TBM are often labeled as smokers after being
“diagnosed” with emphysema. This can lead to insurance companies denying claims on the grounds of “false indication of nonsmoking status.”
*Norwood
TBM Review: A bigger problem than our medical system realizes:
• COPD: >$30 billion annually…
• TBM likely present in 10% of > 12 million COPD patients, whose current treatment is annually greater than $5,000.
TBM is likely contributing significantly to the obstructive physiology in many of these individuals.
• TBM is a progressive disease that can be identified noninvasively through low dose dynamic CT.
• Tracheoplasty has been shown in small trials to substantially improve objective and subjective outcomes.
An opportunity…
• Given the extraordinary chronic costs of “COPD” therapy, and the great potential for localized defect correction, it is possible that tracheoplasty represents a rare opportunity to both reduce medical costs and improve clinical outcome.
• Further studies are needed to define who would benefit from TBM diagnostics and therapeutic intervention.
Study Proposal
• Involving the following investigators:
– Internists/Pulmonologists
– Radiologists
– Thoracic Surgeon
Initial Enrollment
• Internists/Pulmonologists:
– Identify TBM candidates to be selected for treatment.
– Candidates would be selected from a pool of
COPD/asthmatics with long history of modest treatment benefit.
– Enrollment of individuals with dyspnea, great difficulty clearing secretions and barking cough will be emphasized.
– A functional pulmonary baseline will be established.
Radiologic Diagnosis
• Radiologists:
– Conduct low dose dynamic CT to identify
TBM patients.
– Defect will be described in terms of level,
% obstruction and presence/severity of air trapping.
Treatment phase
• Thoracic Surgeon:
– Of population with TBM defined by dynamic
CT, ½ will undergo corrective tracheoplasty.
– ½ will have “dummy” procedure.
– Patients, internists and follow up radiologists will be blinded to procedure status.
Data Collection
• Following procedure, blinded patients will provide 5 years of subjective data.
• Blinded radiologists will perform postoperative dynamic
CT to assess objective status of TBM 3 months after surgery.
• The patient’s respective internists/pulmonologist will measure objective outcomes for 5 years.
• Strict attention will be paid to cost of care.
• The following endpoints of the control and treatment arms will be compared:
– subjective activity and wellness levels
– exacerbation frequency/severity
– % tracheal/bronchial obstruction, presence and severity of air trapping
– PFT scores
– Need for oxygen
– mortality
– cost of care
• Thank You!
References:
•
Gilkeson, RC, Ciancibello, LM, Hejal, RB, et al Tracheobronchomalacia: dynamic airway evaluation with multidetector
CT. AJR Am J Roentgenol 2001;176,205-210
•
Zhang, J, Hasegawa, I, Feller-Kopman, D, et al Dynamic expiratory volumetric CT imaging of the central airways: comparison of standard-dose and low-dose techniques. Acad Radiol 2003;10,719-724
• Boiselle, PM, Feller-Kopman, D, Ashiku, S, et al Tracheobronchomalacia: evolving role of dynamic multislice helical CT.
Radiol Clin North Am 2003;41,627-636
• Zhang, J, Hasegawa, I, Hatabu, H, et al Frequency and severity of air trapping at dynamic expiratory CT in patients with tracheobronchomalacia. AJR Am J Roentgenol 2004;182,81-85
• Jokinen, K, Palva, T, Sutinen, S, et al Acquired tracheobronchomalacia. Ann Clin Res 1977;9,52-57
•
Ikeda, S, Hanawa, T, Konishi, T, et al Diagnosis, incidence, clinicopathology and surgical treatment of acquired tracheobronchomalacia. Nihon Kyobu Shikkan Gakkai Zasshi 1992;30,1028-1035
•
Carden KA, Boiselle PM, Waltz DA, Ernst A Tracheomalacia and tracheobronchomalacia in children and adults: an indepth review. Chest. 2005 ;127(3):984-1005
•
Baroni RH, Ashiku S, Boiselle PM Dynamic CT Evaluation of the central airways in patients undergoing tracheoplasty for tracheobronchomalacia. AJR Am J Roentgenol. 2005;184(5):1444-9
• Wright CD, Hermes GC, Hammoud ZT, Wain JC, Henning GA, Zaydfudim V, Mathisen DJ Tracheoplasty for expiratory collapse of central airways. Ann Thorac Surg. 2005 Jul;80(1):259-66
• Mannino DM, Braman S The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac
Soc. 2007 Oct 1;4(7):502
• Viegi G Epidemiology of chronic obstructive pulmonary disease (COPD). Respiration. 2001;68(1):4-19.
• Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117:5S –9S
• Halpern MT, Stanford RH, Borker R. The burden of COPD in the U.S.A.: results from the Confronting COPD survey.
Respir Med 2003;97:S81
–S89