Document 15958091
advertisement

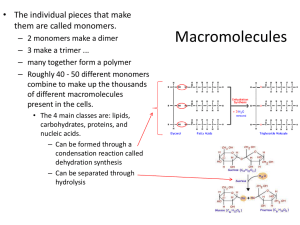
Macromolecules are polymers made through dehydration synthesis (reaction that removes a molecule of water). Examples of macromolecules are: Carbohydrates – sugars Proteins- amino acids Lipids- fatty acids and glycerol Nucleic acids – nucleotides 2 Composed of monosaccharaides, disaccharides and polysaccharides. Monosaccharaides: simple sugars that contain a ratio of C:H:O of 1:2:1 Examples glucose, fructose and galactose Disaccharides: composed of 2 monosaccharaides Examples sucrose, maltose and lactose Polysaccharides: chain of 3 or more monosaccharaides. Starch, glycogen and cellulose 3 Sugars with free carbonyl groups are called reducing sugars. They cause the copper (Cu ²⁺) in the Benedict’s reagent to become reduced (gain electrons) to form Cu ¹⁺, forming Cu₂O . The reaction occurs when the mixture is heated to 100 °C. Before the addition of Benedict’s reagent water glucose sucrose starch milk apple juice potato 4 After the addition of the Benedict’s reagent Water glucose sucrose starch milk apple potato juice juice After the application of heat for 3 minutes. RESULTS: Blue= negative Red = high concentration Orange and yellow = medium concentration Green = low concentration - + - - + + + 5 Starch is a polysaccharide consisting of many glucose monomers linked together into long branching chains. It is the primary storage carbohydrate in plants. In the presence of iodine (I₂-KI) a solution containing starch will turn blue-black in color. The test tube rack contains substances to be tested. water glucose sucrose starch milk apple juice potato juice 6 Blue-black = positive Yellow = negative water glucose sucrose starch milk apple juice potato juice 7 Some water, 10 drops of oil, 5 drops of Sudan IV were added to a tube. Detergent water was added to the test tube and mixed. Detergent is an emulsifier. It surrounds the oil droplets and allows them to stay suspended in the water. The suspended oil droplets stained with Sudan IV give color to the solution. This is an emulsion. After the test tube sits, the oil will separate from the water. 8 In the presence of proteins, biuret reagent reacts with the peptide bonds between the amino acids changing in color from light blue to violet. The intensity of the violet color is proportional to the protein concentration. Biuret reagent does not react with free amino acids. Solutions tested for proteins 9 Light blue= negative Violet = positive End 10