簡單蒸餾裝置對照圖↑
advertisement

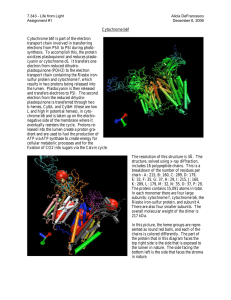
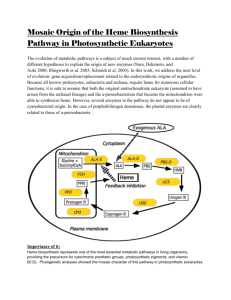
實驗裝置 簡單蒸餾裝置對照圖↑ 此裝製為簡單蒸餾裝製 分餾裝置對照圖↑ 此裝製為分餾裝置 血紅素 C Heme C differs from heme B in that the two vinyl side chains of heme B are replaced by covalent, thioether linkages to the apoprotein. These linkages do not allow the heme C to easily dissociate from the holoprotein, cytochrome c, compared with the more easily dissociated heme B that may dissociate from the holoprotein, the heme-protein complex, even under mild conditions. This allows a very wide range of cytochrome c structure and function, with the myriad of c type cytochromes acting primarily as electron carriers. Cytochromes c, existing as dozens to hundreds of structural and functional subtypes, are found in a wide variety of cell types, even in those organisms living under extreme conditions. While heme B plays a variety of roles in cells, heme C is almost always located in proteins functioning in electron transport reactions most often associated with cell, mitochondria or chloroplast membranes. Heme C, along with the closely structurally related Heme B, are the most common heme types observed in functioning hemeproteins. The thioether linkages are commonly arranged with two amino acids between the two cysteinyl groups bound to the heme. This is often written as CXXC, within the amino acid sequence presentation with X being most any common amino acid. Vertebrate c type cytochromes also arrange a histidine amino acid next to this sequence so that CXXCH represents the five key amino acids associated with the thioether linkages in these proteins. The two thioether bonds are made to heme ring positions 2 and 4, but a few c type cytochromes have hemes with only a single thioether bond.[1] The number of heme C units bound to a holoprotein is highly variable. For vertebrate cells one heme C per protein is the rule but for bacteria this number is often 2, 4, 5, 6 or even 16 heme C groups per holoprotein. It is generally agreed the number and arrangement of heme C groups are related and even required for proper holoprotein function. For instance, those proteins containing several heme C groups are involved with multiple electron transfer reactions, particularly important is the 6 electron reduction required to reduce atmospheric nitrogen into two organic ammonia molecules. It is common for the heme C to amino acid ratio to be high for bacterial hemeproteins, so the interiors of some cytochrome c proteins appear packed with many heme C groups compared with other hemeproteins. Some hemeproteins, often from single cell organisms, may contain five hemes C[2]. The bc1 complex is another important enzyme that contains a C type heme. The thioether linkages seem to allow a great freedom of function for the holoproteins. In general, the c type cytochromes can be "fine tuned" over a wider range of oxidation-reduction potential than cytochromes b. This may be an important reason why cytochrome c is nearly ubiquitous throughout life. Heme C also plays an important role in apoptosis where just a few molecules of cytoplasmic cytochrome c, which must still contain heme C, leads to programmed cell death.[3] In addition to these covalent bonds, the heme iron is also usually coordinated to two side chains of amino acids, making the iron hexacoordinate. For example, mammalian and tuna cytochrome c contain a single heme C that is coordinated to side chains of both histidine and methionine.[4] Perhaps because of the two covalent bonds holding the heme to the protein the iron of heme C is sometimes ligated with the amino group of lysine or even water. The correct structure of heme C was published, in mid 20th century, by the Swedish biochemist K.-G. Paul. [5] This work confirmed the structure first inferred by the great Swedish biochemist Hugo Theorell. The structure of heme C, based upon NMR and IR experiments of the reduced, Fe(II), form of the heme, was confirmed in 1975[6]. The structure HS CH3 H3C H3C CH3 N N Fe N SH CH3 N H3C HO 對照圖↑ O O OH Heme C Identifiers CAS number 26598-29-8 PubChem 444125 MeSH heme+C Properties Molecular formula C34H36O4N4S2Fe Molar mass 684.651 g/mol Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100kPa) Infobox references 3D 作圖: