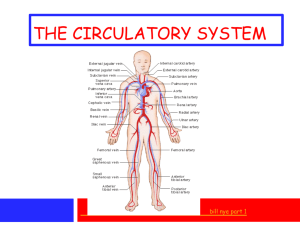
Hemoglobin Oxygen binds reversibly to the iron atoms in the heme... oxide and carbon monoxide as well. Nitric oxide relaxes walls...
advertisement

Hemoglobin Oxygen binds reversibly to the iron atoms in the heme groups. Transport nitric oxide and carbon monoxide as well. Nitric oxide relaxes walls of blood vessels to lower blood pressure. It can bind to specific cysteine residues in hemoglobin and the irons in heme groups. Carbon monoxide will replace the oxygen in the heme groups to form stable complexes that are hard to remove which suffocates the surrounding blood cells because it blocks normal oxygen binding and transport. Four chains of hemoglobin falls apart without the protective casing of the red blood cell. Small changes occur int eh structure of the protein chain when an oxygen binds to the heme. The change will change the shape of the other chains making it easier to bind to oxygen. When oxygen level drops, carbon dioxide levels increase to make hemoglobin release the oxygen causing another domino effect to release the other oxygens. The specific amino acid chain is important because of the way the protein is structured by that chain. In sickle cell anemia, glutamate 6 in the beta chain is changed to a valine which allows deoxygenated hemoglobin to stick to one another making the hemoglobin fibers inside red blood cells stiff. The red blood cell will then become deformed to a fragile state which often breaks and leads to the loss of the hemoglobin. 2 structures denoted by R and T; R for relaxed and has an affinity towards oxygen and T for tense. Heme makes contact with 16 amino acid side chains from seven segments of the chain. Most of the side chains are hydrocarbons except for two histidines. In T structure last amino acid on each chain forms a salt bridge with neighboring subunits. Salt bridge=positive charged N and negative charged O. Most striking difference between the two structures is the width of the gap between the two beta chains. In the T structure, the chains are widely separated and the opening between them is lined with amino acid side chains carrying positive charges to fit DPG and to compensate the negative charge so DPG binding adds more salt bridges to T structure. In the R structure, the gap is narrow so DPG drops out. In oxyhemoglobin, the heme iron is bound o six atoms: 4 nitrogens of the porphyrin to neutralize the iron's charge, one nitrogen on the proximal histidine which links the heme to an alpha helix, and one of the two atoms in the oxygen molecule. In deoxyhemoglobin, the oxygen position is empty, so the heme is bound to only five atoms. In the pocket, if the last amino acid residue, tryosine, is squeezed out of the pocket, the salt bridge would be broken. Therefore, in each pocket a salt bridge is broken, so when enough salt bridges are broken to make the T structure unstable, it reverts back to the R structure. When the histidine and the iron is moved away from the porphyrin, the oxygen molecule would not be able to move due to the porphyrin nitrogens, so the ironoxygen bond breaks.