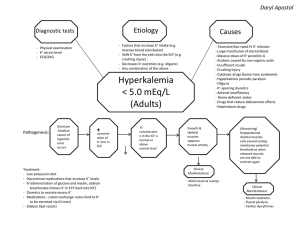
Fluid and Electrolyte Management of the Surgical Patient Hashmi
advertisement

Fluid and Electrolyte Management of the Surgical Patient Hashmi ANATOMY OF BODY FLUIDS • • • • Total Body Water Intracellular Fluid Extracellular Fluid Osmotic Pressure Total Body Water • constitutes 50-70 % of total body weight • fat contains little water, the lean individual has a greater proportion of water to total body weight than the obese person • total body water as a percentage of total body weight decreases steadily and significantly with increasing age Total Body Water Body Water ICF ECF Intravascular Interstitial % of Body Weight % of Total Body Water 60 40 20 4 16 100 67 33 8 25 Intracellular Fluid • largest proportion in the skeletal muscle • potassium and magnesium are the principal cations • phosphates and proteins the principal anions Extracellular Fluid • interstitial fluid: two types – functional component (90%) - rapidly equilibrating – nonfunctioning components (10%) - slowly equilibrating • connective tissue water and transcellular water • called a “third space” or distributional change • sodium is the principal cation • chloride and bicarb the principal anions Osmotic Pressure • physiologic and chemical activity of electrolytes depend on three factors: – the number of particles present per unit volume (moles or millimoles [mmol] per liter) – the number of electric charges per unit volume (equivalents or milliequivalents per liter) – the number of osmotically active particles or ions per unit volume (osmoles or milliosmoles [mOsm] per liter) Terminology • mole: molecular weight of that substance in grams mole eg: sodium chloride is 58 g (Na–23, Cl–35) • equivalent: chemical combining activity; atomic weight expressed in grams divided by the valence – divalent ions (calcium or magnesium) 1 mmol equals 2 mEq • osmole: used when the actual number of osmotically active particles present in solution is considered • millimole of sodium chloride, which dissociates nearly completely into sodium and chloride, contributes 2 mOsm NORMAL EXCHANGE OF FLUID AND ELECTROLYTES • Water Exchange • Salt Gain & Losses Water Exchange • daily water gains – normal individual consumes 2000 to 2500 mL water per day – approximately 1500 mL taken by mouth – rest is extracted from solid food, either from the contents of the food or as the product of oxidation Water Exchange • daily water losses – 250 mL in stools, 800 - 1500 mL in urine, and 600 mL as insensible loss – total losses ~ 2.2 liters – Insensible loss: skin (75%) and lungs (25%) • increased by hypermetabolism, hyperventilation, and fever • 250 mL/day per degree of fever • unhumidified tracheostomy with hyperventilation = insensible loss up to 1.5 L/day Water Exchange Minimum of 500 to 800 mL urine per day required to excrete the products of catabolism Salt Gain and Losses • daily salt intake varies 3-5 gm as NaCl • kidneys excretes excess salt: can vary from < 1 to > 200 mEq/day • Volume and composition of various types of gastrointestinal secretions • Gastrointestinal losses usually are isotonic or slightly hypotonic • should replace by isotonic salt solution CLASSIFICATION OF BODY FLUID CHANGES • Volume Changes • Concentration Changes • Composition Changes – Acid/Base Balance – Potassium Abnormalities – Calcium Abnormalities – Magnesium Abnormalities Volume Changes • If isotonic salt solution is added to or lost from the body fluids, only the volume of the ECF is changed, ICF is relatively unaffected • If water is added to or lost from the ECF, the conc. of osmotically active particles changes – Water will pass into the intracellular space until osmolarity is again equal in the two compartments Volume Changes • BUN level rises with an ECF deficit of sufficient magnitude to reduce GFR • creatinine level may not incr. proportionally in young people with healthy kidneys • hematocrit increases with an ECF deficit and decreases with ECF excess • sodium is not reliably related to the volume status of ECF – a severe volume deficit may exist with a normal, low, or high serum level Volume Deficit • ECF volume deficit is most common fluid loss in surgical patients • most common causes of ECF volume deficit are: GI losses from vomiting, nasogastric suction,diarrhea, and fistular drainage • other common causes: soft-tissue injuries and infections, peritonitis, obstruction, and burns Volume Deficit • signs and symptoms of volume deficit: • CNS: sleepy, apathy – stupor, coma • GI: dec food consumption – N/V • CVS: orthostatic, tachy, collapsed veins - hypotension • Tissue: dec skin turgor, small tongue – sunken eyes, atonia Volume Excess • Iatrogenic or Secondary to renal insufficiency, cirrhosis, or CHF • signs & symptoms of volume excess: – CNS: none – GI: edema of bowel – CVS: elevated CVP, venous distension – pulmonary edema – Tissue: pitting edema – anasarca Concentration Changes • Na+ primarily responsible for ECF osmolarity • Hyponatremia and hypernatremia s&s often occur if changes are severe or occur rapidly • The concentration of most ions within the ECF can be altered without significant osmolality change, thus producing only a compositional change – Example: rise of potassium from 4 to 8 mEq/L would significantly effect the myocardium, but not the effective osmotic pressure of the ECF Hyponatremia (water intoxication) • acute symptomatic hyponatremia (< 130) • hypertension can occur & is probably induced by the rise in intracranial pressure • signs & symptoms: – CNS: twitching, hyperactive reflexes – inc ICP, convulsions, areflexia – CVS: HTN/brady due to inc ICP – Tissue: salivation, watery diarrhea – Renal: oliguria - anuria Hyponatremia (water intoxication) • Hyponatremia occurs when water is given to replace losses of sodium-containing fluids or when water administration consistently exceeds water losses • Hyperglycemia: glucose exerts an osmotic force in the ECF and causes the transfer of cellular water into the ECF, resulting in a dilutional hyponatremia Hypernatremia (water deficit) • The only state in which dry, sticky mucous membranes are characteristic • sign does not occur with pure ECF deficit alone • signs & symptoms: – CNS: restless, weak - delirium – CVS: tachycardia - hypotension – Tissue: dry/sticky muc membranes – swollen tongue – Renal: oliguria – Metabolic: fever – heat stroke Composition Changes • • • • Acid/Base Balance Potassium Abnormalities Calcium Abnormalities Magnesium Abnormalities Acid-Base Balance • large load of acid produced endogenously as a by-product of body metabolism • acids are neutralized efficiently by several buffer systems and subsequently excreted by the lungs and kidneys • Buffers: – proteins and phosphates: primary role in maintaining intracellular pH – bicarbonate–carbonic acid system: operates principally in ECF Acid-Base Balance • buffer systems consists of a weak acid or base and the salt of that acid or base • Henderson-Hasselbalch equation, which defines the pH in terms of the ratio of the salt and acid: – pH = pK + log BHCO3 / H2CO3 = 27 mEq/L / 1.33 mEq/L = 20 / 1 = 7.4 – As long as the 20:1 ratio is maintained, regardless of the absolute values, the pH will remain at 7.4 Acid-Base Balance • Four types of acid-base disturbances • combinations of respiratory and metabolic changes may represent: • compensation for the initial acid-base disturbance or, • two or more coexisting primary disorders • 10-mmHg PaCO2 change yields a 0.08 pH change Respiratory Acidosis • retention of CO2 secondary to decreased alveolar ventilation • management involves prompt correction of the pulmonary defect, when feasible, and measures to ensure adequate ventilation • prevention: tracheobronchial hygiene during the postoperative , humidified air, and avoiding oversedation Respiratory Alkalosis • PaCO2 should not be below 30 mmHg • dangers of a severe respiratory alkalosis are those related to potassium depletion – hypokalemia is related to entry of potassium ions into the cells in exchange for hydrogen and an excessive urinary potassium loss in exchange for sodium • shift of the oxyhemoglobin dissociation curve to the left, which limits the ability of hemoglobin to unload oxygen at tissues Metabolic Acidosis • Anion gap is a useful aid: • normal value is 10 to 15 mEq/L • unmeasured anions that account for the “gap” are sulfate and phosphate plus lactate and other organic anions • measured ions are sodium, bicarb, and chloride Metabolic Acidosis • treatment of metabolic acidosis should be directed toward correction of the underlying disorder • sodium bicarbonate is discouraged, attempt to treat underlying cause • shifts the oxyhemoglobin dissociation curve left • interference with O2 unloading at the tissue level Metabolic Alkalosis • common surgical patient has hypochloremic, hypokalemic metabolic alkalosis resulting from persistent vomiting or gastric suction in the patient with pyloric obstruction • unlike vomiting with an open pylorus, which involves a combined loss of gastric, pancreatic, biliary, and intestinal secretions Pathophysiology of Paradoxic Aciduria occurring with GOO • GOO -> hypochloremic, hypokalemic, metabolic alkalosis • urinary bicarb excretion to compensate for alkalosis • volume deficit progresses aldosterone-mediated sodium resorption is accompanied by potassium excretion • kidneys primary goal becomes volume preservation sodium resorption • either K+ or H+ must be excreted to keep a balanced • due to already excessive hypokalemia, the kidney excretes H+ in place of K+, producing paradoxic aciduria Potassium Abnormalities • normal daily dietary intake of K+ is approx. 50 to 100 mEq • majority of K+ is excreted in the urine • 98% of the potassium in the body is located in ICF @ 150 mEq/L and it is the major cation of intracellular water • intracellular K+ is released into the extracellular space in response to severe injury or surgical stress, acidosis, and the catabolic state Hyperkalemia • signs & symptoms: – CVS: peaked T waves, widened QRS complex, and depressed ST segments Disappearance of T waves, heart block, and diastolic cardiac arrest – GI: nausea, vomiting, diarrhea (hyperfunctional bowel) Hypokalemia • K+ has an important role in the regulation of acid-base balance • alkalosis causes increased renal K+/H+ excretion • signs & symptoms: – CVS: flatten T waves, depressed ST segments – GI: paralytic ileus – Muscular: weakness - flaccid paralysis, diminished to absent tendon reflexes Calcium Abnormalities • majority of the 1000 to 1200g of calcium in the average-sized adult is found in the bone • Normal daily intake of calcium is 1 to 3 gm • Most is excreted via the GI tract • half is non-ionized and bound to proteins • ionized portion is responsible for neuromuscular stability Hypocalcemia • signs & symptoms (serum level < 8): – numbness and tingling of the circumoral region and the tips of the fingers and toes – hyperactive tendon reflexes, positive Chvostek's sign, muscle and abdominal cramps, tetany with carpopedal spasm, convulsions (with severe deficit), and prolongation of the Q-T interval on the ECG Hypocalcemia • causes: • acute pancreatitis, massive soft-tissue infections (necrotizing fasciitis), acute and chronic renal failure, pancreatic and small-bowel fistulas, and hypoparathyroidism Hypercalcemia • signs & symptoms: – CNS: easy fatigue, weakness, stupor, and coma – GI: anorexia, nausea, vomiting, and weight loss, thirst, polydipsia, and polyuria Hypercalcemia • two major causes: • hyperparathyroidism and cancer – bone mets – PTH-like peptide in malignancies Magnesium Abnormalities • total body content of magnesium 2000 mEq • about half of which is incorporated in bone • distribution of Mg similar to K+, the major portion being intracellular • normal daily dietary intake of magnesium is approximately 240 mg • most is excreted in the feces and the remainder in the urine Magnesium Deficiency • causes: – starvation, malabsorption syndromes, GI losses, prolonged IV or TPN with magnesium-free solutions • signs & symptoms: – similar to those of calcium deficiency Magnesium Excess • Symptomatic hypermagnesemia, although rare, is most commonly seen with severe renal insufficiency • signs & symptoms: – CNS: lethargy and weakness with progressive loss of DTR’s – somnolence, coma, death – CVS: increased P-R interval, widened QRS complex, and elevated T waves (resemble hyperkalemia) – cardiac arrest Secretions FLUID AND ELECTROLYTE THERAPY • Preoperative Fluid Therapy • Intraoperative Fluid Therapy • Postoperative Fluid Therapy Preoperative Fluid Therapy • Correction of Volume Changes: Volume deficits result from external loss of fluids or from an internal redistribution of ECF into a nonfunctional compartment – nonfunctional because it is no longer able to participate in the normal function of the ECF and may just as well have been lost externally • Correction of Concentration Changes: If severe symptomatic hypo or hypernatremia complicates the volume loss, prompt correction of the concentration abnormality to the extent that symptoms are relieved is necessary Postoperative Fluid Management • replace losses & supply a maintenance: – open abdomen losses: 8 cc/kg/hr – NGT & urine output – Blood loss x 3 – Replace with isotonic salt solution (LR or NS) – unwise to administer potassium during the first 24 h, until adequate urine output has been established even a small quantity of potassium may be detrimental because of fluid shifts Fluid Composition Fluid Replacement Status Acute Renal Failure • Classified according to its cause: • Prerenal: hypotension, hypovolemia, renal artery occlusion/stenosis, cardiac failure • Renal: trauma, toxins (contrast, endotoxin), drugs (NSAIDS, aminoglycosides), pigment (myoglobin, hemaglobin) • Postrenal: ureteral obstruction, bladder dysfxn (anesthesia, meds, nerve injury), urethral obstruction, foley obstruction Laboratory Studies • Urinalysis: blood or myoglobin is a positive diagnostic test - can test via Hemoccult card • Urinary lytes: urine sodium, creatinine, urea, osmolality, and specific gravity help classify type of renal failure using Renal failure indices Renal Indices Indices U Osm U/P osm U/P urea U/P cr Urine Na Prerenal > 500 >1.25 >8 > 40 < 20 Renal < 350 <1.1 <3 < 20 > 40 Postrenal Varies Varies Varies < 20 > 40 FENa < 1% > 3% > 3% Indications for use of Dialysis in Acute Renal Failure • • • • • Severe acidosis Electrolyte abnormalities Inability to clear toxins Volume overload Uremic signs and symptoms (encephalopathy, BUN > 100)