Homework Chapters 3 and 4 With Answers
advertisement

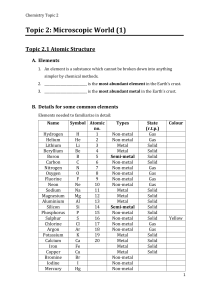
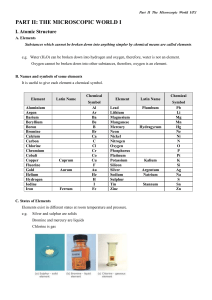
Homework Chapters 3 and 4 With Answers 1.Give the symbol and name of the element in group 5A and period 3. How many electrons are in outer shell of this element? How many valence electrons does this element have? Is the element a metal, non-metal or metalloid? How many electron shells are occupied by electrons? P, phosphorus, 5, 5, non-metal, 3 2. Give an example of a transition element in period 4. What would be the most likely number of electrons (valence electrons) in the outer shell of these elements? Zinc (or 9 other possibilities), 2 3. For each pair of metal atoms below, which would have the lowest IP (ionization potential/ionization enery)? Which would be the larger atom? Which would be most reactive? a. Na and K K for all three b. Al and Mg Mg for all three 4. List atoms of the following elements from smallest to largest. C K Mg Ne O Si Ne < O < C < Si < Mg < K 5. In general terms, where in the periodic table are the most reactive metals? Bottom Left The most reactive non-metals? Top Right except inert gases The most unreactive elements? Group VIIIA 6. How do the reactivities in question 5 relate to the size of the atoms of the elements? Larger metals and smaller non-metals are more reactive 7. Draw Lewis structures for atoms of: a. Ca (with 2 dots) b. H (with one dot) c. P (with 5 dots) d. Ar (with 8 dots) 8. Represent the predicted ions that each of the following atoms would form? i.e., an ion of aluminum would be Al3+. a. Pb b. Ca c. S d. Ne a. Pb4+ b. Ca2+ c. S2- d. Ne0 (i.e., doesn’t tend to form an ion) 9. Predict and write the correct name and formula for a compound of each of the following. [OK to use table 4.7 as an aid] a. Na and Cl (NaCl) b. Ca and Br (CaBr2) c. Mg and O (MgO) d. Al and the nitrate ion Al(NO3)3 e. a compound composed of 2 N atoms and one O atom. (N2O) 10. Give the name of these compound formulae. a. K2O b. ZnSO4 c. Fe2O3 d. P2O5 e. HCl f. Mg3(PO4)2 a. potassium oxide b. zinc sulfate c. Iron(III) oxide d. diphosphorus pentoxide e. hydrogen chloride f. magnesium phosphate 11. Draw Lewis structures for the following compounds. a. Ammonia - NH3 b. Methane - CH4 c. formaldehyde - CH2O (use C as a central atom) d. Carbon dioxide – CO2 e. hydroxide ion - OH1- f. HCN (use C as a central atom) (will show in class) 12. Which of each pair below would have the highest boiling point or melting point? a. H2O or Br2 b. NaCl or H2O c. C3H8 or C2H6O d. C4H10 or C3H8 a. H2O b. NaCl c. C2H6O d. C4H10 13. Determine whether each of the following would be ionic or covalent? a. BaSO4 b. KCl c. N2O d. H2SO4 e. H2O f. NH4Cl a. ionic b. ionic c. covalent d. covalent e. covalent f. ionic - even though made of all nonmetals, the ammonium ion IS an ion. This is an uncommon situation rather unique to the ammonium ion.