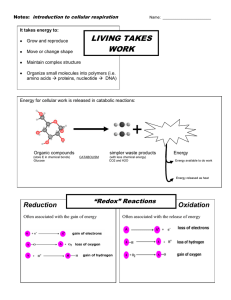
Metabolism Lecture 5, part 1 Fall 2008
advertisement

Metabolism Lecture 5, part 1 Fall 2008 Metabolism Metabolism • All the biochemical process within an organism that maintain life and contribute to growth • Emergent properties – The whole is greater than the sum of its parts – New properties emerge with each step upward in the hierarchy of life • Cellular metabolism arises from interactions between molecules within the orderly environment of the cell 1 Metabolism • Metabolic pathway – Series of chemical reactions • Catabolic pathway – Breaks down a complex molecule into simpler compounds – Creates energy • Anabolic pathway – Builds a complex molecule from simpler compounds – Consumes energy • Bioenergetics – Study of how energy flows through living organisms 2 Energy Energy • The capacity to cause change • The ability to rearrange a collection of matter Two main types • Kinetic energy • Potential energy 3 Energy Kinetic energy • Energy of motion – Does work by imparting motion to other objects • Thermal energy (heat) – Type of kinetic energy – Amount of energy associated with the random movement of atoms and molecules – Temperature • Measure of how much thermal energy a molecule possesses • The faster the molecule, the more collisions, the higher the temperature 4 Energy Potential energy • Stored energy • Based on location or structure • Chemical energy – Form of potential energy available for release in a chemical reaction 5 Chemical Energy Chemical energy • Energy stored in chemical bonds – Released when bonds between molecules broken • Produces heat, kinetic energy and waste products • Amount of kinetic energy produced is how efficient the process is – Car: 25% kinetic energy – rest is lost as heat – Cellular respiration: 40% cellular work, rest is used for body heat 6 7 Review Chemical Bonds, pg. 38-41 • Covalent • Ionic • Hydrogen 8 Energy Transformation • Energy can change from potential to kinetic and back 9 Energy Transformation • Energy can change from potential to kinetic and back Fig. 8.2 Energy & Thermodynamics Thermodynamics • Study of energy transformations that occur in a collection of matter First Law of Thermodynamics (Principle of Conservation of Energy) – Energy is neither created nor destroyed, only converted from one form to another – Amount of matter & energy in the universe remains the same – Energy is always conserved • Can be converted from one form to another. e.g. photosynthesis converts energy from the sun into plant biomass. • Energy quantity stays the same Fig.8.3 10 Energy & Thermodynamics Second Law of Thermodynamics – When energy is changed from one form to another, some of the useful energy is always degraded to lower-quality, more dispersed, less useful energy. • Usually heat – e.g. cellular respiration glucose + oxygen = carbon dioxide + water + energy (+ heat) • Energy quality is changed Fig.8.3 11 Energy & Entropy • Entropy – amount of disorder in a group of molecules – Heat - high disorder, high entropy, less useful form of energy Second Law of Thermodynamics (revisited) • Every energy transformation increases the entropy of the universe • Cells are not disordered – use energy to fight entropy • Organisms are “islands of low entropy in an increasingly random universe” 12 How do chemical reactions happen? • Reactants – starting materials • Products – resulting materials • • Balanced equations Matter is neither created nor destroyed, only rearranged – Breaking and forming of chemical bonds 13 14 How do chemical reactions happen? 3H2 + N2 → 2NH3 ← • Reaction is reversible • Chemical equilibrium – A dynamic but stable state of a reversible chemical reaction in which the forward reaction and reverse reaction proceed at the same rate, so that the concentrations of reactants and products remain constant – Changing chemical equilibrium • Changing concentration of reactants or products • Changes in temperature (e.g., gas to liquid) How do chemical reactions happen? What makes a chemical reaction spontaneous? – Proceed on their own without any continuous external influences (energy) Reactions tend to be spontaneous if: 1. the products have lower potential energy than the reactants 2. when the product molecules are less ordered than the reactant molecules 15 16 What makes a chemical reaction spontaneous? 1. Reactions tend to be spontaneous if the products have lower potential energy than the reactants • Products have lower potential energy if their electrons are held more tightly than electrons of reactants • More electronegative – Electronegativity – The tendency of an atom to attract electrons towards itself 17 What makes a chemical reaction spontaneous? • Enthalpy (ΔH) – Measure of difference in energy between reactants and products – When reaction is exothermic ΔH is negative • Exothermic – Chemical reaction that releases heat • Endothermic – Chemical reaction that absorbs heat What makes a chemical reaction spontaneous? 2. Reactions tend to be spontaneous when the product molecules are less ordered than the reactant molecules • Entropy (S) – amount of disorder in a group of molecules – Δ S is positive when products are less ordered than reactants – Spontaneous reactions increase entropy 18 19 Free Energy • Physical and chemical process proceed in direction that results in lower potential energy (negative ΔH) and increased disorder (positive ΔS) Gibbs free-energy change (ΔG) • Free energy – The portion of a system’s energy that can perform work when temperature and pressure are uniform throughout the system (e.g., living cell) ΔG = ΔH - T ΔS • T= temperature in Kelvin – Temperature becomes more important in determining free-energy change as the temp of molecules increases Fig. 8.5 20 Free Energy ΔG = ΔH - T ΔS • If ΔG is less than 0, reaction is spontaneous = exergonic – Net release of free energy • If ΔG is greater than 0, reaction is not spontaneous = endergonic – Absorbs free energy from its surroundings – Stores free energy in molecules Fig. 8.6 21 Free Energy & Equilibrium • When ΔG is zero, reactions are at equilibrium – Free energy decreased – Systems cannot spontaneously move away from equilibrium • Living cells not at equilibrium – Products become reactants in other metabolic pathways ATP & Cellular Work Three main types of work • Mechanical – E.g. moving cilia, contracting muscles • Transport – Transport of molecules across cell membrane • Chemical – Promoting chemical reactions that do not happen spontaneously (endergonic) Most cellular work done by ATP 22 23 ATP & Cellular Work ATP (adenosine triphosphate) • Adenine (nitrogenous base) • Ribose (sugar) • 3 phosphate groups – Phosphate groups negatively charged – Repelling of charges = high potential energy • Unstable molecule – Hydrolysis breaks bond of terminal phosphate group – Products: Adenosine diphosphate (ADP) and inorganic phosphate – Exergonic: releases energy • 7.3 kcal per mole ATP Fig. 5.5 24 ATP & Cellular Work Phosphate Transfer • Phosphorylation – Transfer of the phosphate group from ATP to some other molecule • This phosphorylated molecule undergoes a change that performs work – More reactive/less stable – Conformation change – Phosphorylated = molecule that receives the phosphate group Fig. 8.11 25 ATP & Cellular Work Energy coupling • Transfer of energy from processes that yield energy (exergonic) to processes that consume energy (endergonic) Fig. 8.10 26 ATP & Cellular Work ATP recycling • ATP used continuously by organism • ADP+ inorganic P brought together again via cellular respiration – Very rapid - 10 million ATP molecules spent & regenerated per second per active muscle cell Fig. 8.12