Chem 161 Ready for class? Ch1 to 4 Review
advertisement

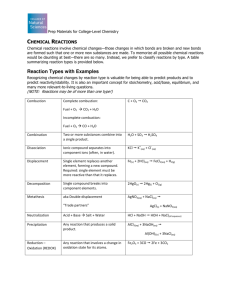
Chem 161 Ready for class? Ch1 to 4 Review 1. Sketch a periodic table. On it, label a) representative elements b) transition metals c) halogens d) alkaline earth metals e) semi-metals (metalloids) 2. a) List all diatomic molecules. b) List all elements that are gaseous at room temperature. c) List all elements that are liquid at room temperature. d) Using the information above, write a balanced equation for I) Nitrogen reaction with oxygen to make dinitrogen pentoxide (g). II) Making water from ‘scratch’. III) Decomposition of MgO(s) . 3 . What is the formula of a compound with a) sodium and chlorine? What is the name of this compound? b) magnesium and sulfur? What is the name of this compound? C) nitrate and aluminum? What is the name of this compound? d) sulfate and hydrogen? What is the name of this compound? 3. A) Is density intensive or extensive property? b) What does it mean to be intensive? c) Name two other properties that are intensive. 4. There are 4 significant figures in 0.04102cm with the estimation in 2, and it means anywhere between 0.04101cm to 0.04103cm. Answer the following using this example. a) There are ____ significant figures in 12.50g with the estimation in ________ , and it means anywhere between ________________________. b) There are ____ significant figures in 100 g with the estimation in ________ , and it means anywhere between ________________________. c) There are ____ significant figures in 100. g with the estimation in ________ , and it means anywhere between ________________________. 5. A) Briefly state the significant figure rules when adding or subtracting. Show an example. b) Briefly state the significant figure rules when multiplying or dividing. Show an example. 6. Give three examples of a) element b) molecular compound c) cation d) ionic compound 7. A) If you have to ‘mix’ hydrogen and oxygen to make H2O, why is H2O considered a compound, not a mixture? b) Both hydrogen and oxygen are diatomic and should be written as H2 and O2. If this is the case, why is water H2O? Not H2O2? 8. Each ‘box’ of the periodic table has three information. The whole number on top is called ________________ and the decimal number on the bottom is called ________________ with the unit of ________________. 9. (a) Mole (mol) is a name given for the number 6.022x1023. If you have 2 mol water molecules, it means you have _________________ water molecules. However if you are counting elements, there are ________________ elements since each water molecule has ___ elements. b) Mole (mol) is a name given for the number 6.022x1023. If you have 5 mol Ca(OH)2, it means you have ______________________ Ca(OH)2. However if you are counting ions, there are ________________ ions since each Ca(OH)2 has ___ ions, and if you are counting just the anions, there are _______________anions since each Ca(OH)2 has ____ anions. 10. (a) One the average, 6.022 x 1023 helium weigh 4.0g. We may write this as ( 4.0g / 6.022 x 1023 helium ) or (4.0g / 1 mol He ). Using this, you can say 3 mol He weighs _______ g, and 40.g of He is equivalent to _____ mol of He. (b) One the average, 6.022 x 1023 oxygen gas weigh 32 g . We may write this as ( 32g / 6.022 x 1023 O2 ) or (32g / 1 mol O2 ). Using this, you can say 4.5 mol O2 weight _______ g and 97g of O2 is equivalent to _______ mol O2. 11. What is the molar mass for (please include unit!) a) ammonium phosphate? b) carbonic acid? b) nitrogen gas? 12. P4 (s) + 10 Cl2 (g) 4 PCl5 (g) a) This equation reads, 1 molecule of P4 react with 10 molecules of Cl2 to produce 4 molecules of PCl5. How many molecules of Cl2 do you need to react with 5 molecules of P4? Clearly show your work. b) This equation reads, 6.22x1023 (1mol) molecule of P4 react with 6.22x1024 (10 mol) molecules of Cl2 to produce 2.4088x1023 (4mol) molecules of PCl5. How many mole of PCl5 would be produced from 6.0mol of of Cl2? Clearly show your work. c) How many grams of product would you get from 350.g of Cl2? Show your work clearly!! d) What is the amount of P4 (s) that you had to ASSUME to have in (C)? e) Are you calculating the ‘theoretical’ or ‘actual” yield in (c)? 13. You have two solutions : 500.mL of 2.0M NaCl and 500mL of 2.0M CO2. a) Is NaCl an ionic compound or a molecular compound?. Explain your answer. b) Is CO2 an ionic compound or a molecular compound?. Explain your answer. c) Calculate the number of moles of NaCl in 500.mL of 2.0M NaCl. d) Calculate the number of moles of CO2 in 500mL of 2.0M CO2. e) What type of compound is NaCl? What type of compound is CO2? f) Calculate the number of moles of “things” in 500.mL of 2.0M NaCl. Think about what is actually present in water. Is it NaCl? Na and Cl? Na+ and Cl-? Na and Cl2?... g) Calculate the number of moles of “things” in 500mL of 2.0M CO2. Think about what is actually present in water. Is it CO2? C and O? C and O2?..... 14. How many mL of 0.446 M KMnO4(aq) are required to react with 50.0 mL of 0.200 M H2C2O4(aq) ? 2 KMnO4(aq) + 5 H2C2O4(aq) + 3 H2SO4(aq) 2 MnSO4(aq) + 10 CO2(g) + 8 H2O + K2SO4(aq) 15 . Assign oxidation number for… Show your work/logic. a) Mn in KMnO4 b) C in C3H8O c) vanadium in (NH4)3VO4 d) carbon in K2C2O4 e) boron in Na2B4O7 16. Identify each reaction to be oxidation, reduction, redox, or non-redox. Show your work/logic. a. Ba2+(aq) + CrO42-(aq) BaCrO4(s) b. 2 H+(aq) + CO32-(aq) H2O(l) + CO2(g) c. Fe3+(aq) Fe2+(aq) d. MnO2(s) MnO4-(aq) e. 2 CrO42-(aq) + 2 H+(aq) Cr2O72-(aq) + H2O(l) 17. The following reaction describes the production of metallic iron: 2Fe2O3 + 3C 4Fe + 3CO2 How many electrons are lost by each carbon atom in this reaction? Show your work/logic. 18. Consider the redox equation, C4H10(l) + Cr2O72-(aq) + H+(aq) H6C4O4(s) + Cr3+(aq) + H2O(l) The oxidizing agent is … 19. The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs which element would undergo oxidation most readily? Based on this list, 20. Using the activity series given above, predict the products or predict ‘no reaction. a) Al+3 (aq) + Mg(s) b) Al(s) + Mg+2(aq) c) AlCl3 (aq) + Mg(s) d) Al(s) + MgCl2 (aq) e) Compare reactions (a) and (b). f) Compare reactions (a) and (c). g) Compare reactions (b) and (d). 21. When the carbohydrate, C12H22O11, undergoes complete combustion (products are CO2 and water), a) write a balanced equation b) Above the balanced equation, assign oxidation number for EACH atom of each element in the equation. c) the reducing agent in the reaction is… 22. When the carbohydrate, C12H22O11, undergoes incomplete combustion (products are CO and water), a) write a balanced equation b) the reducing agent in the reaction is.. c) Compare these answers to question above and explain the condition that might lead to incomplete combustion. 23. BrO3-(aq) + Zn(s) + Br-(aq) + Zn2+(aq) a) Assign oxidation numbers for each element. Be clear. b) Reducing agent? c) Oxidizing agnet? d) How many electrons do you think should be transferred once balanced correctly? Show your work/logic. Figure it out without balancing the whole equation. 24. C4H10(l) + Cr2O72-(aq) + H+(aq) H6C4O4(s) + Cr3+(aq) + H2O(l) a) Assign oxidation numbers for each element. Be clear. b) Reducing agent? c) Oxidizing agnet? d) How many electrons do you think should be transferred once balanced correctly? Show your work/logic. Figure it out without balancing the whole equation.