Membrane Biophysics Membrane Protein
advertisement
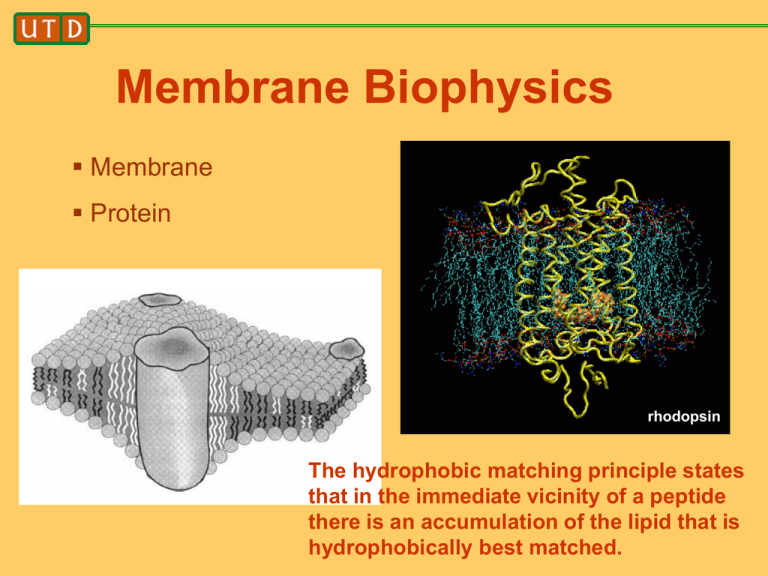
Membrane Biophysics Membrane Protein rhodopsin The hydrophobic matching principle states that in the immediate vicinity of a peptide there is an accumulation of the lipid that is hydrophobically best matched. Outline • Focus on model systems – Experimental model systems – Theoretical model systems • Model systems do not have full biological complexity • Their value is in being able to do careful studies to both discover and test conceptual ideas, which hopefully are biologically relevant. • Main topic: hydrophobic matching Biological membranes: different cellular organelles have different lipid and protein membrane compositions. Why not study organelle membranes in their full complexity? Cells determine the bilayer characteristics of different membranes by tightly controlling their lipid composition. We still have only sketchy information on the lipid composition of organellar membranes. In addition, we know little about lipid–protein interactions at the molecular level, let alone, lipid– lipid interactions in complex mixtures. Nat. Rev. Mol. Cell Biol. 2:504–513 (2001). Protein – lipid interactions • Focused on model systems • What is the simplest possible model system? • Answer: make the bilayer membrane with only one type of lipid, and use a synthetic, designed peptide. J. Antoinette Killian. Professor of biophysical chemistry of membranes at Utrecht University acetyl-GWWL(AL)nWWA-ethanolamide n=3 -- 8 Hydrophobic matching principle Hydrophobic interactions play a major role in stabilizing membrane structures. Transmembrane peptides usually have a hydrophobic region, flanked by aromatic and hydrophilic residues. Peptides are rigid compared to lipids FEBS Letters 458 (1999) 271-277 Hydrophobic matching principle Exposure of protein hydrophobic residues to water, or of lipid hydrophobic groups to water, is unfavorable. What changes will minimize the free energy? 1. 2. 1. Lipids adapt, forming a meniscus 2. Peptide is expelled from the membrane Why might 2. be favored over 1.?? Answer: it costs energy to distort a membrane. How much energy? Related to the bending modulus, compression modulus, etc. if we think of the membrane as an elastic sheet (continuum description). Slope gives area expansion modulus Molecular dynamics simulations are used in order to study the self-assembly process and the physical properties of flexible membranes composed of amphiphilic molecules. On molecular scales, these membranes are observed to be rather mobile and to have rough surfaces arising from molecular protrusions, i.e., from the relative displacements of individual molecules. On length scales that are only somewhat larger than the membrane thickness, on the other hand, the membranes are found to undergo smooth bending undulations. In this way, our study provides the first explicit connection between computer simulations with molecular resolution and elastic membrane models based on differential geometry. bending modulus Phys. Rev. Lett. 82, 221, (1999). Micropipette aspiration Science 284, 1143 (1999). (C and D) Microdeformation of a polymersome. The arrow marks the tip of an aspirated projection as it is pulled by negative pressure, DP, into the micropipette. Aspiration acts to (i) increase membrane tension, t = 1/2 DPRp/(1 - Rp/Rs), where Rp and Rs are the respective radii of the micropipette and the outer spherical contour; and (ii) expand the original, projected vesicle surface area, Ao, by the increment DA. Protein-Induced Bilayer Deformations, and LipidInduced Protein Tilting These are some of the possibilities with a singlecomponent lipid bilayer. For bilayers composed of more than one type of lipid, there are many more possibilities! Some geometries of lipid phases packed spheres Micellar cubic phase I1 packed cylinders hexagonal phase H1 packed planes lamellar phase La cubic phases important for membrane protein crystallization blue, green, red regions are water stacked bilayers – why? Get better signal to noise in x-ray diffraction studies TEM image of a multilamellar vesicle onion Caffrey, M. 2000. A lipid’s eye view of membrane protein crystallization in mesophases. Curr. Opin. Struct. Biol. 10:486–497. Caffrey, M. 2000. A lipid’s eye view of membrane protein crystallization in mesophases. Curr. Opin. Struct. Biol. 10:486–497. Electron density map constructed from x-ray diffraction data. Yang, L., and H. W. Huang. 2002. Observation of a membrane fusion intermediate structure. Science. 297:1877– 1879. More generally, inverted phases can be induced in phospholipid membranes by dehydration, heating, the addition of divalent cations, and the addition of lipids of negative intrinsic curvature. closely related to membrane fusion Peptide-induced La to inverted phase transition B A S. O. Nielsen et. al., Biophys. J. 87, 2107 (2004) in explanation of experimentally observed transition at 30:1 lipid:short-peptide concentration. C A. meniscus forms around peptides. B. water fills the meniscus regions. C. head groups rearrange to solvate the water pores. Structure of the inverted phase 6:1 lipid to peptide concentration in HII phase. Long peptides do not cause this transition. Biochemistry 35, 1037 (1996) Biological context: membranes are composed of many lipid species. The hydrophobic matching principle states that in the immediate vicinity of a peptide there is an accumulation of the lipid that is hydrophobically best matched. Lipids come in different shapes, which determines the self-assembled structures they prefer to form (bilayers, micelles, inverse micelles) Why would a biological membrane contain non-bilayer forming lipids? Answer: for vesicle budding and membrane fusion Nat. Rev. Mol. Cell Biol. 2:504–513 (2001). Nat. Rev. Mol. Cell Biol. 2:504–513 (2001). The organelles along the exocytic and endocytic transport routes are connected by carrier vesicles that bud from one organelle and fuse with the next. We will introduce the new and important concept of protein/lipid sorting in membranes. It is suggested that in any membrane, the hydrophobic mismatch inherent to the protein and lipid composition may be released by a process of protein aggregation or, more interestingly, via a general mechanism of protein/lipid sorting. This concept of hydrophobic mismatch-dependent protein/lipid sorting is particularly attractive due to its inherent self- organizing character. Nature Reviews in Molecular Cell Biology, volume 2, page 504 (2001). Sphingolipid domains sort proteins. A membrane that contains mostly sphingomyelin, with or without cholesterol, is thicker than one composed of phosphatidylcholine and cholesterol, which is in turn thicker than a membrane of phosphatidylcholine alone. This implies that sphingolipid–cholesterol domains are thicker than the surrounding membrane. Cells probably use this feature to sort membrane proteins that are destined for the plasma membrane from Golgi proteins by the length of their transmembrane domains. For example, the transmembrane domains of plasma membrane proteins are 20 residues long, whereas those of Golgi proteins are only 15 residues long. Discrete increases in membrane thickness would allow the sorting of various populations of membrane protein. Hydrophobic mismatch induces formation of a meniscus S. O. Nielsen et. al., Biophys. J. 87, 2107 (2004) S. O. Nielsen et. al., Biophys. J. 88, 3822 (2005) • Maximal possible change in first shell lipid length is small and represent only a partial response to mismatch. (consistent with experiments, slope < 1) • Membrane thickens at intermediate range • The zero mismatch length can be obtained and compared to results obtained from free-energy calculations. Free Energy Profile of Membrane Meniscus against Hydrophobic Mismatch 1 DF (u0 ) k (u0 u0opt ) 2 2 Lipids do influence protein function—the hydrophobic matching hypothesis revisited (Biochimica et Biophysica Acta 2004, 1666, 205) Xibing He, S.O. Nielsen, et. al., manuscript in preparation: first time this effect has been quantified by computer simulation k = 650 kJ/mol h0 1/ 2 1/ 4 3 / 4 ( ) K L 2 Lipid tilting J. Chem. Phys., 119, 7435 (2003) Lipid tilting in the vertical direction • • • Shorter tubes make the lipids adopt a more tilted and disorganized configuration (compared to bulk). Longer tubes make lipids acquire a straighter configuration. Straightening at intermediate distance due to thickening of membrane. Lipid tilting Lipid tilting in the planar direction • • For all peptide lengths the lipid’s head-totail vector points away from the peptide (due to rigid peptide, consistent with theory) with the shortest peptide giving the biggest tilting. Region of negative correlation due to void resulting from positive meniscus (also seen by B. Smit) The polar-aromatic residues Trp and Tyr have a specific affinity for a region near the lipid carbonyls. Porin from the outer membrane of Rhodobacter capsulatus (a) and the membrane-bound form of the gramicidin A dimer (b). Trp and Tyr residues are shown as spacefilling models. Trp The membrane/water interfacial region is a chemically complex environment and offers opportunities for interactions with amino acid side chains by dipole-dipole, charge, H-bonding, etc. Tyr What do 3-d structures say? A central hydrophobic section is bordered on both sides by aromatic belts, which were proposed to interact favorably with the lipid headgroups. Statistical analysis of porins and helix-bundle membrane proteins such as cytochrome c oxidase shows that Trp and Tyr (but not Phe) are concentrated at the membrane/water interface. Charged residues are found only outside the aromatic belts (aqueous environment). Phe Trp What chemical properties would be responsible for a preferred localization of aromatic and charged amino acid side chains near the interfacial region? Tyr Must interact with a polar/apolar interface Trp has a large fused aromatic ring which might be buried in the hydrophobic part of the bilayer. The amide group might localize in the more polar environment at the interface. The amide group can act as a hydrogen bond donor. Phe Tyr is smaller but has a similar ring system. Phe is aromatic, but is completely hydrophobic and is found in the transmembrane part of membrane proteins. Lys and Arg can snorkel Arg Lys Biophysical approach which allows a direct comparison between the interfacial interactions of different amino acid side chains in transmembrane peptides: WALP: GWW(LA)nLWWA KALP: GKK(LA)nLKKA These peptides are incorporated into pure lipid model membranes (ie. one lipid type only) Results suggest that Trp is anchored rather rigidly to the interface, whereas Lys is much more flexible and can be accommodated in a larger range of bilayer thicknesses than Trp. Although biophysical studies have the obvious advantage that detailed and fairly direct structural interpretations can often be made, they are quite far removed from the complexities of a biological membrane, and their relevance for understanding what happens in vivo can be questioned. However, the available methods for studying membrane proteins in vivo rarely allow measurements to be made with the precision required for locating individual amino acids relative to the membrane–water interface. There is one method that works in vivo: you will be asked about this on your take-home exam. What is the biological relevance of these findings? The strength of interfacial interactions might influence the conformational flexibility of multi-spanning membrane proteins. E. coli membrane channel protein TolC Relatively rigid proteins that require no or only very subtle structural changes for functioning, such as gramicidin or porins, might be conveniently and stably anchored by Trp. If functional properties require changes in tilt or conformation, more flexible anchors, at least on one side of the membrane, might be more suitable. This would be in agreement with the recent suggestion for single span membrane proteins that Trp fulfills a stabilizing role as interfacial anchoring residue, in particular at the trans-side of the membrane, whereas Lys, as topological determinant, remains preferentially at the cis-side (“positive-inside rule”), where it can act as a flexible anchor.




