Chapter 8 Chemical and Physical Change: Energy, Rate, and

Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 8
Chemical and
Physical Change:
Energy, Rate, and
Equilibrium
8.1 Thermodynamics
• Thermodynamics - the study of energy, work, and heat.
– applied to chemical change
– applied to physical change
• The laws of thermodynamics help us to understand why some chemical reactions occur and others do not.
• As the bonds are broken and new bonds are formed, energy is required or released.
• We can measure the change in energy during these changes.
• System - the process under study
– Usually the chemical reaction or physical change of interest.
• Surroundings - the rest of the universe.
• We will be able to measure the change in energy in the form of heat as the temperature changes.
The Chemical Reaction and Energy
• Important points to kinetic molecular theory
– molecules and atoms in a reaction mixture are in constant, random motion;
– these molecules and atoms frequently collide with each other;
– only some collisions, those with sufficient energy, will break bonds in molecules; and
– when reactant bonds are broken, new bonds may be formed and products result.
Exothermic and Endothermic Reactions
•
The first law of thermodynamics
– the energy of the universe is constant, E cannot be created nor destroyed.
1
• Where does the energy come from that is released and where does the energy go when it is absorbed?
• The chemical bond is stored chemical energy.
• If the energy required to form new bonds > the energy released when the old bonds are broken, there will need to be an external supply of energy…
Endothermic reaction .
A-B + C-D
A-D + C-B
These bonds must be broken.
This releases energy.
These bonds are formed.
This requires energy
•
Enthalpy - represents heat energy.
• Change in Enthalpy ( D
H o ) - energy difference between the products and reactants.
• Energy released (exothermic), enthalpy change is negative
(energy diagram).
• Energy absorbed (endothoermic), enthalpy change is positive
(energy diagram).
Entropy
•
The second law of thermodynamics - the universe spontaneously tends toward increasing disorder or randomness.
•
Entropy (S o ) - a measure of the randomness or disorder of a chemical system.
•
High entropy - highly disordered system
•
Low entropy - well organized system
•
No such thing as negative entropy.
D
S o of a reaction = S o
(products) - S o
(reactants)
• A positive D
S o means an increase in disorder for the reaction.
• A negative D
S o means a decrease in disorder for the reaction.
Spontaneous and Nonspontaneous Reactions
• Spontaneous reaction - occurs without any artificial external energy input.
• Often, but not always, exothermic reactions are spontaneous.
• Thermodynamics is used to help predict if a reaction will occur.
• If exothermic and positive ΔS o = Spontaneous
• If endothermic and negative ΔS o = Nonspontaneous
• For other situations, it depends on the relative size of ΔH o and ΔS o .
•
Free energy (
D
G
o
)
- represents the combined
2 contribution of the enthalpy and entropy values for a chemical reaction.
D
G o =
D
H o - T
D
S o
T in Kelvins
• Predicts spontaneity
• Negative D
G o …Spontaneous
• Positive D
G o …Nonspontaneous
8.2 Experimental Determination of Energy
Change in Reactions
• Calorimetry - the measurement of heat energy changes in a chemical reaction.
• The change in temperature is used to measure the heat loss or gain.
• Calorie – the quantity of heat required to raise 1 g of water
1 °C.
• Nutritional Calorie (large Calorie) = 1kilocalorie (1kcal) or
1000 calorie.
•
Specific heat (S.H.) - the number of calories of heat needed to raise the temperature of 1 g of the substance 1 o C.
• S.H. for water is 1.0 cal/g o C
• To determine heat released or absorbed, need:
– specific heat
– total number of grams of solution
– temperature change (increase or decrease)
Q
m
D
T
S.H.
8.3 Kinetics
4
• Thermodynamics determines if a reaction will occur but tells us nothing about the time it will take.
•
Kinetics - the study of the rate of chemical reactions.
– Also gives the mechanism - step-by-step description of how reactants become products.
• We will look at:
– disappearance of reactants and
– appearance of products.
Let’s consider the following reaction:
CH
4
(g) + 2O
2
(g)
CO
2
(g) +2H
2
O(g) + 211 kcal
• C-H and O=O bonds must be broken and C=O and O-H bonds must be formed
• Energy is required to break the bonds.
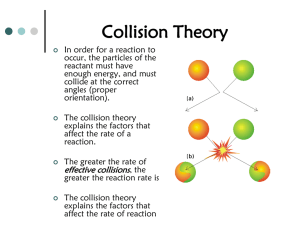
– Comes from the collision of the molecules.
– Effective collision - one that leads to a chemical reaction.
• Activation energy - the minimum amount of energy required to produce a chemical reaction.
•
Activated complex - extremely unstable complex, the formation of which requires energy.
Factors That Affect Reaction Rate
• Structure of the reacting molecules,
• Concentration of reactants,
• Temperature of reactants,physical state of reactants, and
• Presence of a catalyst
Structure of Reacting Molecules
• Oppositely charged species react more rapidly
• Ions with the same charge do not react.
• Bond strength plays a role.
• Magnitude of the activation energy is related to bond strength
• Size and shape influence the rate.
• Large molecules may block the reactive part of the molecule.
The Concentration of Reactants
• Rate will generally increase as concentration increases .
• Caused by a greater number of collisions
The Temperature of Reactants
• Rate increases as the temperature increases.
• Higher temp. means higher K.E.
• Higher K.E. means higher percentage of these collisions will result in product formation.
Physical State of Reactants
• Solid state:
• atoms, ions or molecules are close but restrictive in motion.
• Gaseous state:
• particles are free to move but are far apart causing collisions to be relatively infrequent.
• Liquid state:
• particles are free to move and are close together.
• The typical order of rate per state of reactant?
• Liquid > gas> solid
Presence of a Catalyst
• Catalyst - a substance that increases the reaction rate.
• Catalysts interact with the reactants to create an alternative pathway for product formation by lowering the activation energy.
• Enzyme - a biological catalyst that controls and speeds up thousands of essential biochemical reactions.
8.4 Equilibrium
Rate and Reversibility of Reactions
• Equilibrium reactions - chemical reactions that do not go to completion (incomplete reactions).
8
• After no further obvious change, measurable quantities of reactants and products remain.
•
Reversible reaction - a process that can occur in both directions.
– Use the double arrow symbol
•
Dynamic equilibrium - the rate of the forward process is exactly balanced by the rate of the reverse process.
Equilibrium
• Example: Sugar in Water
– If put 2-3 g of sugar in 100 mL water, all will dissolve.
– sugar (s) sugar (aq)
• If dissolving 100 g in 100 mL of water, not all of it will dissolve.
– Over time, you observe no further change in the amount of dissolved sugar.
– Individual sugar molecules are constantly going into and out of solution and happens at the same rate.
• The double arrow serves as
– an indicator of a reversible process
– an indicator of an equilibrium process, and
– a reminder of the dynamic nature of the process.
• Equilibrium constant (K eq
)- ratio of the two rate constants .
sugar(s) sugar(aq)
The Generalized Equilibrium-Constant
Expression for a Chemical Reaction
a A + b B c C + d D
K eq
[ C] c
[ D] d
[A] a
[ B] b
9
Writing Equilibrium-Constant Expressions
• Each chemical reaction has a unique equilibrium constant value at a specified temperature.
• The brackets represent molar concentration.
• All equilibrium constants are shown as unitless.
• Only the concentration of gases and substances in solution are shown.
• concentration for pure liquids and solids are not shown.
Interpreting Equilibrium Constants
•
The value of Equil. constant tells us the extent to which reactants have converted to products.
1. K eq greater than 1 x 10 2 .
• Large value of K eq
: numerator > denominator.
2. K eq
• At equilibrium mostly product present.
less than 1 x 10 -2 .
• Small value of K eq
: numerator < denominator.
• At equilibrium mostly reactant present.
3. K eq between 1 x 10 -2 and 1 x 10 2 .
•
Equilibrium mixture contains significant concentration of both reactants and products.
• LeChateleir’s Principle
- if a stress is placed on a system at equilibrium, the system will respond by altering the equilibrium composition in such a way as to minimize the stress.
We will examine the following “stresses.”
1. Effect of Concentration
2. Effect of Heat
3. Effect of Pressure
4. Effect of Catalyst
1. Effect of Concentration
N
2
(g) + 3H
2
(g) 2NH
3
(g)
• Adding or removing reactants and products at a fixed volume.
• Addition of N
2 or H
2
. To minimize the stress, which way will the reaction shift?
• To the right. Forming more products.
• If NH
3 is put in the reaction vessel?
• Equilibrium shifts to the left, forming more reactants.
2. Effect of Heat
• Exothermic reactions : treat heat as a product
N
2
(g) + 3H
2
(g) 2NH
3
(g) + 22 kcal
• Addition of heat is similar to increasing the amount of product. If heat is generated, which way will the equilibrium shift? To the left.
• Endothermic Reaction - treat heat as a reactant.
39 kcal + 2N
2
(g) + O
2
(g) 2NH
3
(g)
• Which way will this reaction shift if the reaction is heated?
To the right.
3. Effect of Pressure
• Pressure affects the equilibrium only if one or more substances in the reaction are gases
• Relative number of gas moles on reactant and product side must differ.
• When pressure goes up…shift to side with less moles of gas. When pressure goes downs…shifts to side with more moles of gas.
4. Effect of a Catalyst
• A catalyst has no effect on the equilibrium composition. It increases the rate of both the forward and reverse reaction to the same extent.