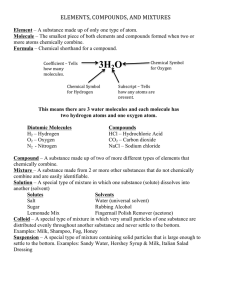
Elements Mixtures Compounds
advertisement
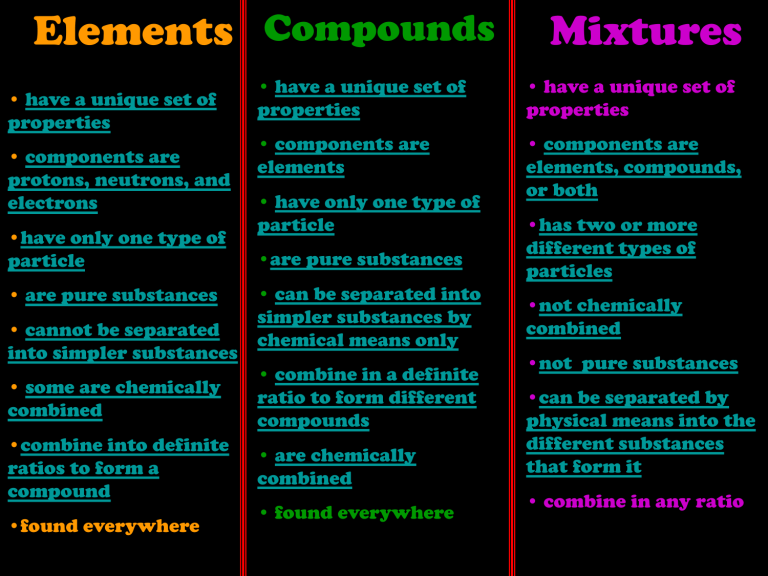
Elements Compounds • have a unique set of properties • components are protons, neutrons, and electrons •have only one type of particle • are pure substances • cannot be separated into simpler substances • have a unique set of properties • have a unique set of properties • components are elements • components are elements, compounds, or both • have only one type of particle •are pure substances • can be separated into simpler substances by chemical means only • some are chemically combined • combine in a definite ratio to form different compounds •combine into definite ratios to form a compound • are chemically combined •found everywhere Mixtures • found everywhere •has two or more different types of particles •not chemically combined •not pure substances •can be separated by physical means into the different substances that form it • combine in any ratio Pure Substance is a substance in which there is only one type of particle; includes elements and compounds. Definite Ratio Combinations of Elements forming Compounds H2O2 2 atoms of hydrogen to one of oxygen 2 atoms of hydrogen to 2 of oxygen CO2 1 atom of carbon to 2 of oxygen carbon dioxide CO 1 atom of carbon to 1 of oxygen carbon monoxide H2O C6H12O6 NaCl CH2O 6 atoms of carbon, to 12 of hydrogen, to 6 of oxygen 1 atom of sodium to 1 of chlorine 1 atom of carbon, to 2 of hydrogen, to 1 of oxygen water hydrogen peroxide sugar salt wood Element A pure substance that cannot be separated into simpler substances by physical or chemical means. hydrogen carbon silicon ELEMENT PURE SUBSTANCE Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Na Metals Metalloids • shiny • some are shiny some are dull • malleable • ductile • good conductors of thermal and electric energy • have reactivity • some are malleable and some are not • some are ductile and some are not •semiconductors of electric energy • have reactivity Nonmetals • dull • brittle • not malleable • not ductile • poor conductors of thermal and electric energy • some have reactivity Compound A pure substance composed of two or more elements that are chemically combined. + Molecule of the Compound Water + hydrogen Chemically combined hydrogen _ oxygen Mixture Mixture A combination of two or more substances that are not chemically combined. Sodium chloride and water molecules chloride sodium + chloride sodium + COMPOUND WATER PURE SUBSTANCE MIXTURE of TWO ELEMENTS and the COMPOUND WATER NOT A PURE SUBSTANCE oxygen Chemically combined O2 Properties of Compounds • color •shape •boiling point • melting point • density • freezing point • reactivity ( combustible, explosive, and flammable) burning wood CH2O + O2 -----> CO2 + H2O Mixture Compound Element ELEMENT PURE SUBSTANCE COMPOUND WATER PURE SUBSTANCE MIXTURE of TWO ELEMENTS and the COMPOUND WATER NOT A PURE SUBSTANCE Solution The liquid that results when water dissolves a substance. The Compound Water Chemically combined + + H2O - Electrons being shared between atoms of hydrogen and oxygen. sodium chloride + - - + + - + - + + + - + + + - NaCl + Na ion Cl ion sodium metal salt chlorine + gas H is the symbol O is the symbol of Hydrogen for Oxygen H2 O The subscript means each molecule of water has two atoms of hydrogen No subscript after oxygen means each molecule of water has only one atom of oxygen. The coefficient means that there are three molecules of water. molecule 3 H 2O Parts of a Chemical Equation C + O2 yields or produces The compounds or elements on the left side of the arrow are the starting materials, or REACTANTS. CO2 The new substance produced is the PRODUCT. The product is placed to the right of the arrow. A plus sign separates reactants when there is more than one. NaOH + HCl NaCl + H2O A plus sign separates products when there is more than one new substance produced. In a chemical equation the number of atoms in the reactants should always equal to the number of atoms in the products. C + O2 1 atom carbon 2 atoms oxygen = CO2 1 atom 2 atoms carbon oxygen Element A pure substance that cannot be separated into simpler substances by physical or chemical means. Chemically oxygen combined O2 Compound A pure substance composed of two or more elements that are chemically combined. Molecule of the Compound Water + + Mixture A combination of two or more substances that are not chemically combined. Sodium chloride and water molecules chloride hydrogen Chemically combined _ sodium + carbon silicon