SCIE 0900 Review Worksheet ... Extra Homework #3
advertisement
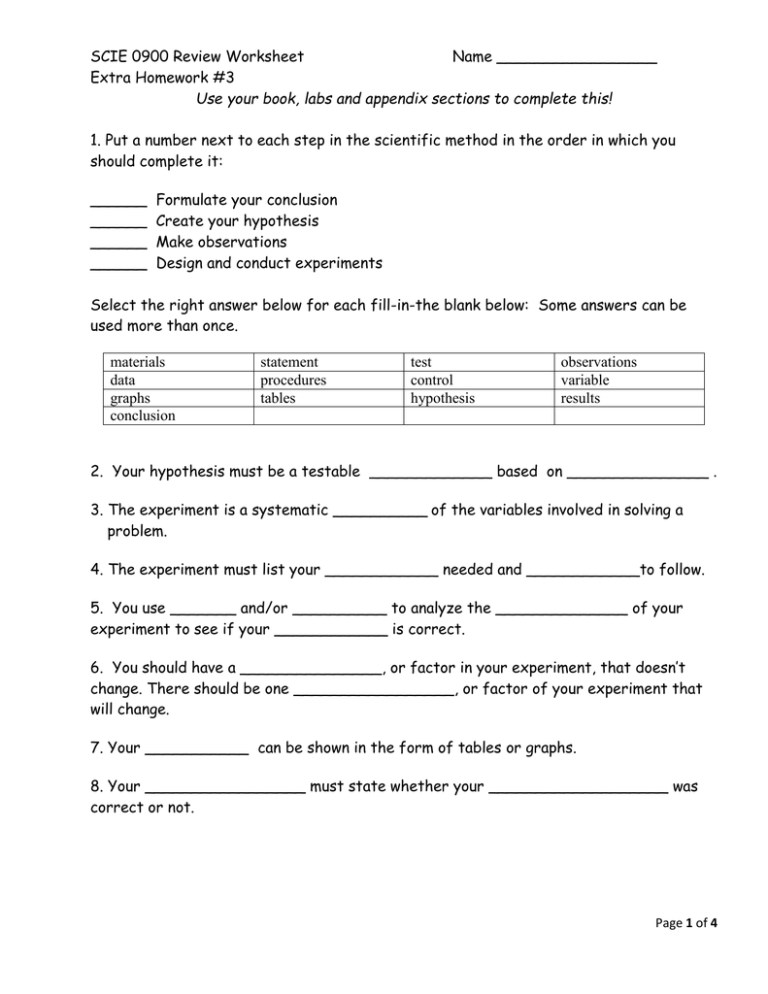
SCIE 0900 Review Worksheet Name _________________ Extra Homework #3 Use your book, labs and appendix sections to complete this! 1. Put a number next to each step in the scientific method in the order in which you should complete it: ______ ______ ______ ______ Formulate your conclusion Create your hypothesis Make observations Design and conduct experiments Select the right answer below for each fill-in-the blank below: Some answers can be used more than once. materials data graphs conclusion statement procedures tables test control hypothesis observations variable results 2. Your hypothesis must be a testable _____________ based on _______________ . 3. The experiment is a systematic __________ of the variables involved in solving a problem. 4. The experiment must list your ____________ needed and ____________to follow. 5. You use _______ and/or __________ to analyze the ______________ of your experiment to see if your ____________ is correct. 6. You should have a _______________, or factor in your experiment, that doesn’t change. There should be one _________________, or factor of your experiment that will change. 7. Your ___________ can be shown in the form of tables or graphs. 8. Your _________________ must state whether your ___________________ was correct or not. Page 1 of 4 Dimensional Analysis Review: 1. Dimensional Analysis uses ______________ factors or unit factors to convert from one metric unit to another. 2. What are the steps for metric conversions using dimensional analysis? 1. Given _____________ with it’s unit 2. Set up the ___________________ factor a. Place the ___________ unit as the denominator of the _________________ factor b. Place the desired unit as the _______________ numerator c. Place a _______ in front of the ____________ unit d. Place a _______ in front of the smaller unit. e. Repeat b-d until reaching the final answer 3. ___________ units - only the desired unit should be left 4. Solve the _______________ 3. Complete the following problems: a. 0.52 cg 1 dg 1 ______ 1 dag 1 _____ ______ kg 1 ______ cg 10 dg 10 ____ _____ dg 10 hg ________ kg b. 456 L 1 10 _____ 1 L _____ cL 10 mL _____ dL 1 cL c. 6750 mm = ____________ hm d. 0.789 kg = _____________ cg 10 10 1 mL 1 _________ µL Page 2 of 4 Energy, Chemistry and Solutions Review 1. The ________________ states that the total amount of energy is a system remains constant. The energy can be ______________ from one form to another, but know new _________ can be created not any existing energy can be _____________. 2. ___________ is the study of matter. 3. Matter is anything that occupies _____________ and has ______________. 4. Matter is made up of _______________. 5. Elements are made up of ____________ which are the smallest particle of an element. 6. Protons and ___________ are in the nucleus of the _________ and the _____________ are in the area around the nucleus. 7. To know which element an __________ belongs to is determined by the number of __________ in the nucleus. 8. An ____________ is a substance that cannot be broke down or transformed into a different substance by chemical processes. 9. A _________________ is a pure substance, made up of 2 or more elements, that can be broke down into 2 or more simpler substances by a chemical reaction. 10. A ________________ is formed when 2 or more _____________ join together chemically. 11. A chemical ___________ is used to represent and element and a chemical ______________ is an abbreviation for a chemical compound. 12. A solution is a _______________ mixture . The parts of a solution are the __________ and the ________________. ______________ is called the universal solvent. Page 3 of 4 13. The rate of dissolving a given solvent can is depended upon 3 factors which are: a. b. c. 14. Using the formula determine the concentrations for the following problems. % solute X 100% solution a. What is the % m/m if 15.0 g of sugar are added to 225 g of water? b. What is the % m/v if 45.2 g of salt are dissolved in enough water for a final volume of 325 mL? c. What is the % v/v if 263.5 mL of alcohol are added to enough water for a total solution of 5000 mL? Page 4 of 4


