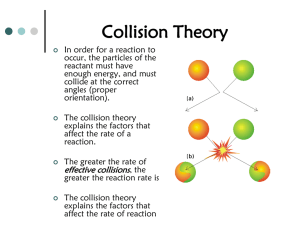
Document 15518965
advertisement

During a chemical reaction heat can be either absorbed or released. › As the reactants become the products energy can be released exothermic › As the reactants become the products energy can be absorbed endothermic The energy levels of the reactants and the products will be different. ΔH = Enthaply The difference in the energy levels of a reaction. ΔHreaction = ΔHproducts – ΔHreactants ΔH is negative for exothermic reactions ΔH is positive for endothermic reactions Many reactions require an input of energy for them to start, this is known as Activation Energy. The ΔH of the reactants, products and activation energy can be graphed. The variables of energy and reaction path/progress are graphed. The amount of energy required to push the reactants over the edge, to start the reaction, is the activation energy. Once at this point the reaction takes place and products are formed. The difference in the energy of the reactants and products is the ΔH In exothermic reactions energy of the products is less than the reactants. Energy is released (heat is given off). ΔH is negative. Endothermic reactions usually have a steep activation energy, because the energy of the products is MUCH higher than the reactants (LOTS of energy input). ΔH is positive (energy absorbed). Another factor influencing reactions is known as Entropy (ΔS). Entropy is a measure of the random disorder in a reaction. With respect to molecules in phases: Phase Amount of Entropy Solid Low Liquid Medium Gas High Enthalpy and Entropy both influence reaction rate. Free energy is a measure of the available energy to do work. ΔG = ΔH - T ΔS ΔG = Free energy ΔH = Enthalpy ΔS = Entropy T = Temperature Spontaneous reactions occur on their own, without any outside energy input. This depends upon ΔS, ΔH and ΔG ΔG Reaction Behavior Negative Spontaneous Zero Equilibrium Positive Will not proceed on its own