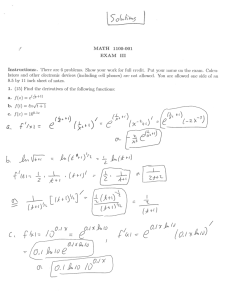
Annual Report for the contained use of Genetically Modified
advertisement

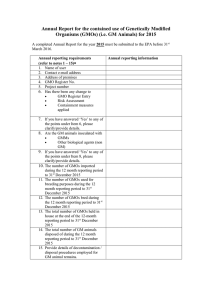
Annual Report for the contained use of Genetically Modified Organisms (GMOs) (i.e. GM Plants) for 2008. A completed Annual Report for the year 2008 must be submitted to the EPA before 31st March 2009. Annual reporting requirements (refer to notes 1 – 7) 1. Name of user 2. Contact e-mail address 3. Address of premises 4. GMO Register No. 5. Project number 6. Has there been any change to GMO Register Entry Risk Assessment Containment measures applied Annual reporting information 7. If you have answered ‘Yes’ to any of the points under Item 6, please clarify/provide details. Notes 1. In addition to the name of the user, please provide the name of the Principal Investigator under whose name the GMO contained use facility/activity is registered. 2. Contact e-mail address such that the Agency may revert to you should further clarification be required. 3. Address of premises where GMO activity is carried on. 4. GMO Register No - This number may be referenced on the GMO Letter of Agreement issued by the Agency in respect of the GMO contained use activity 5. Where a number of different projects exist on your GMO Register Entry, please reference the project to which you are referring. If necessary use a different form for each project number. 6. Respond ‘Yes’ or ‘No’ to each of the three bullet points. 7. Where new GMO project work is being/has been undertaken, a Risk Assessment completed in respect of the new GMO must accompany this form. Please forward form to: GMO Enforcement, Office of Climate, Licensing & Resource Use, Environmental Protection Agency, PO Box 3000, Johnstown Castle Estate, County Wexford.