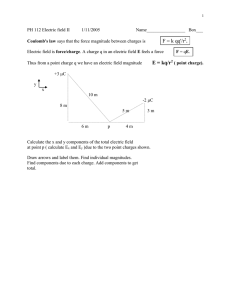
Coulomb’s Law Physics I Class 18 18-1
advertisement

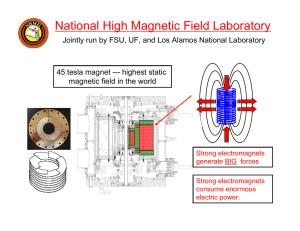
Physics I Class 18 Coulomb’s Law Rev. 25-Mar-04 GB 18-1 Important Announcement About Exam 2 Due to the unexpected level of difficulty of Exam 2, we are taking the unusual step of curving the grades. The score shown on your exam is your raw score. Your curved grade, which we will use to determine your exam average, can be found on WebCT as “Exam 2”. Any grading changes will be made to your raw score and will automatically change your curved score. We used the following formula for the curve: Score 10 Raw (rounded to nearest 0.1 point) 18-2 Forces Known to Physics (Review) There are four fundamental forces known to physics: Gravitational Force (“yesterday’s news”) Electromagnetic Force (start today) Weak Nuclear Force Strong Nuclear Force (All forces we observe are comprised of these fundamental forces. Most forces observable in everyday experience are electromagnetic on a microscopic level.) 18-3 A New Property of Matter Charge Charge comes in two types: positive and negative. NET charge can neither be created nor destroyed. (Principle of Conservation of Charge) However, positive and negative charges can be separated or combined. Neutral H proton electron 0 charge +e charge -e charge Charge is quantized – the smallest unit of charge (magnitude) in normal experience is the charge of the electron or proton, “e”. (All charges are integer multiples of this unit.) By arbitrary historical convention, the charge of an electron is negative and the charge of a proton is positive. 18-4 Conservation of Charge Charge is even conserved in nuclear reactions. Here is what happens to a free neutron (outside a nucleus) in about 12 minutes: neutron proton electron anti-neutrino 0 charge +e charge -e charge 0 charge This is an example of the weak nuclear force (beta decay). 18-5 Coulomb - A Man, A Unit, A Law Charles Coulomb, 1736-1806 Coulomb invented a delicate torsion balance with which he was able to measure the forces between charged and magnetic objects with sufficient accuracy to verify a previous conjecture that the mathematical formula for electromagnetic force should resemble the formula for gravity. The unit of charge is named after Coulomb, abbreviated C. 1.0 C = 6.24150975 × 10+18 e 1.0 e = 1.60217646 × 10–19 C One Coulomb is a lot of protons! 18-6 Coulomb’s Law of Electrostatic Force F 1 q1 q 2 (r̂ ) (Prof. B’s version – more later.) 2 4 0 r The meaning of each term: F: Electrostatic force on charge 1 from charge 2. 1 2 2 : Electrostatic force constant = 8.98755 × 10 +9 N m /C 4 0 q1 : Value of charge 1, positive or negative. q 2 : Value of charge 2, positive or negative. r 2 : Center distance from point charge 1 to point charge 2, squared. r̂ : Unit vector from charge 1 to charge 2. 18-7 Direction of Electrostatic Force “Opposites Attract” 18-8 Properties of Electrostatic Force Similarities with Gravity Every object with charge is attracted or repelled by every other object with charge. (Opposites attract, same repel.) Electric force is a force at a distance (through occupied or empty space). Electric force is a “central” force (center-to-center for point charges). Electric force varies as the inverse square of the center distance. Electric force varies as the product of the charges. 18-9 Properties of Electrostatic Force Differences with Gravity Electrostatic force is both attractive and repulsive, depending on the signs of charge. Gravity is always attractive. There is only one sign of mass and no way to “cancel out” positive mass with negative mass. A charged object can attract an object with no net charge by causing polarization (a Physics 2 topic). The electric force between a proton and an electron is far larger than the gravitational force. (Next slide.) 18-10 Comparison of Gravity and Electrostatic Force r = distance between proton and electron (doesn’t matter) M = mass of a proton = 1.67252 × 10–27 kg. m = mass of an electron = 9.1091 × 10–31 kg. 2 2 G = gravitation constant = 6.673 × 10–11 N m /kg e = charge of proton (+) or electron (–) = 1.60217646 × 10–19 C 1 2 2 = electrostatic constant = 8.98755 × 10+9 N m /C 4 0 Considering only the ratio of the magnitudes: Felec Fgrav 1 e2 4 0 r 2 e2 M m 4 0 G M m G 2 r 2.269 × 10+39 That number is dimensionless – the same everywhere in the universe (as far as we know). A deep mystery: Why is it so large? 18-11 Superposition of Electrostatic Forces +5.0 C +1.0 C Y 1 X resultant -3.0 C N 1 q1 q i Fon 1 ( r̂i ) (find and add X and Y components) 2 i 2 4 0 ri 18-12 Two Ways of Calculating the Electric Force Vector Book Method: Find the magnitude of the force vector using the absolute values of the charges. Use trigonometry and “opposite attract, like repel” to find the direction. Convert to X, Y components. Prof. B’s Method (Used in Computational Electromagnetics) A systematic method that will give you the X and Y components without resorting to trigonometry or intuition. Use whichever method works best for you. 18-13 How to Calculate a Unit Direction Vector From blue (x0,y0) to red (x,y): 1. Find the displacement in X,Y components. d ( x x 0 ) î ( y y 0 ) ĵ 8 î 6 ĵ 2. Find the length of this vector. 3. Divide by the length to get a unit vector. r | d | ( x x 0 ) 2 ( y y 0 ) 2 82 6 2 10 r̂ d r 8î 6 ĵ 10 0.8î 0.6 ĵ A “unit vector” is a special vector with dimensionless length of one unit. Y 6 0 X 0 8 18-14 How to Calculate the Electric Force Vector (Prof. B’s Method) Force on blue charge (x0,y0) from red charge (x,y): 1. Find the value of the scalar part of the formula: 1 q1 q 2 F 4 0 r 2 2. 3. 4. Y (could be + or –) Find the unit vector from blue to red. This will be in X and Y components. Change the sign on both components. (Takes into account like charges repel.) Multiply F from step 1 times each component from step 3 toget final X and Y components of force. Make sure you account for all – signs! 6 0 X 0 8 18-15 Class #18 Take-Away Concepts 1. Charges come in positive and negative. 2. Opposites charges attract, like charges repel. 3. Coulomb’s Law of Electrostatic Force F 1 q1 q 2 (r̂ ) 2 4 0 r 4. Similarities and differences with gravity. 5. The principle of superposition. N 1 q1 q i Fon 1 (r̂i ) 2 i 2 4 0 ri 18-16 Class #18 Problems of the Day Qa Qb Qc d 2.0 cm ___1. Three non-zero point charges are arrayed on a line as shown above. Qa has zero net electric force from Qb and Qc. Qc = +6.4 x 10-19 C and it is 2.0 cm from Qa. What are possible values for Qb and d to two sig. figures? (Select all answers that are possible.) A. B. C. D. Qb = –3.2 x 10-19 C and d = 1.0 cm. Qb = –1.6 x 10-19 C and d = 1.0 cm. Qb = –3.6 x 10-19 C and d = 1.5 cm. Qb = –4.0 x 10-20 C and d = 0.50 cm. 18-17 Answer to Problem 1 for Class #18 The answer is B. This is a bit of a trick question. Answer A does not work numerically unless you forget to square the distance. Answers B, C, and D work numerically. However, C and D are not possible because they violate charge quantization. 18-18 Class #18 Problems of the Day 2. Calculate the value in Coulombs of the total charge in Avogadro’s Number of protons. What would be the attractive force (magnitude) in Newtons on that much positive charge from an equal amount of negative charge one meter distant? 18-19 Answer to Problem 2 for Class #18 The charge is Avogadro’s Number times the charge of one proton: Q N A e (6.02 10 23 )(1.60 10 19 ) 96320 C The magnitude of the force is 1 Q2 9 2 2 19 F ( 8 . 99 10 )( 96320 ) / 1 8 . 34 10 N 2 4 0 r That’s a big force! 18-20 Activity #18 Coulomb’s Law (Another Pencil and Paper Activity) Objective of the Activity: 1. 2. 3. Think about Coulomb’s Law. Consider the implications of the Coulomb’s Law formula. Practice calculating electrostatic forces using superposition. 18-21 Class #18 Optional Material “Three Quarks for Muster Mark” We mentioned earlier that charge is quantized in units of +/– e. The charge of an electron is – e; the charge of a proton is +e. Physicists now accept the Quark Theory, which holds that hadrons (elementary particles that interact with each other through the strong force) are composed of combinations of deeper fundamental particles: quarks. Quarks have charges of +/– 1/3 e and +/– 2/3 e. Let’s see why the Quark Theory was developed and how quarks form the building blocks of protons and neutrons. 18-22 “Elementary” Particles An Embarrassment of Riches Beginning with the discovery of the electron in 1898, physicists encountered an increasing array of so-called “elementary” particles. It became evident to physicists in the 1960’s that these particles must themselves be combinations of deeper fundamental particles. Joseph F. Alward, PhD Department of Physics University of the Pacific 18-23 The Origin of Quark Theory 1929- Murray Gell-Mann took the name quark from "Three quarks for muster Mark", in James Joyce's book Finnegan's Wake. (1963) (Nobel Prize 1969) In the early 1960’s, Gell-Mann and others proposed the Quark Theory to explain the “elementary” particles and their interactions in terms of 3 deeper fundamental particles called quarks. Further developments have shown there are actually 6 different quarks and their corresponding anti-quarks. The quarks and their properties have been given whimsical names like “charm” that have no physical significance. 18-24 6 Quark Building Blocks Quarks Anti-Quarks Anti-Bottom 18-25