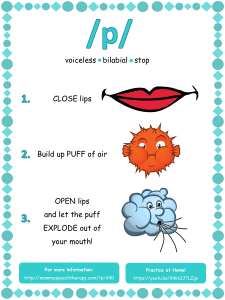
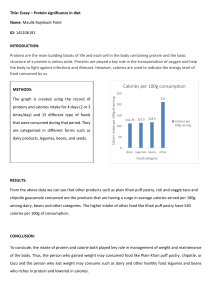
Mr. O Name: Calories
advertisement

Mr. O Calories Name: Cheese Puff Lab Material: Procedure: Data Volume of water ____________ Initial temperature of water____________ Final temperature of Water____________ What is your observation of cheese puff after the experiment? Calculations: 1 Calorie = 4.184 J Calculate the Joules and calories of the cheese puff Analysis Questions 1. How does this experiment illustrate the law of conservation? 2. The cheese puff actually released more energy than our data shows. Explain why. 3. How do companies determine the number of calories a food contains? 4. Base on this lab, and what you learned in biology last year, why do we need top eat food? 5. Why do you need a food in the experiment with a high fat content? What other foods could be tested in this manner?