Balancing Chemical Equations
advertisement

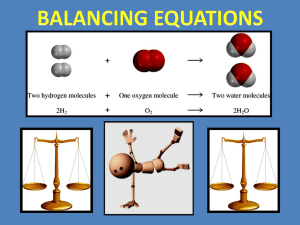
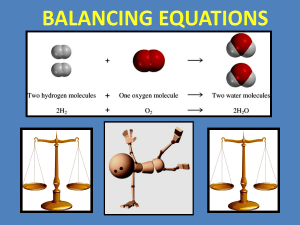
You Should Be Able To… 1. Define and explain the law of conservation of mass 2. Represent chemical reactions and the conservation of atoms, using molecular models 3. Write and balance (using the lowest whole number coefficients) chemical equations from formulae, word equations, or descriptions of experiments Subscript Coefficient Law of Conservation of Mass Molecule Atom Skeleton Equation Balanced Equation Word Equation • Chemical reactions result in chemical changes. – Chemical changes occur when new substances are created. – The original substance(s), called reactants, change into new substance(s) called products. Reactants (c) McGraw Hill Ryerson 2007 Products See pages 202 - 203 Reactants (c) McGraw Hill Ryerson 2007 Products See pages 202 - 203 • Chemical reactions can be written in different ways. – A word equation: • Nitrogen monoxide + oxygen nitrogen dioxide – A symbolic equation: • 2NO(g) + O2(g) 2NO2(g) COEFFICIENTS STATE OF MATTER - Letters indicate the state of each compound. (aq) = aqueous/dissolved in water - Indicates how many of each molecule there is. (s) = solid -Ie: there are 2 molecules of NO. (g) = gas (c) McGraw Hill Ryerson 2007 ( ) = liquid • When a chemical reaction occurs, new compounds are created, BUT… – No new matter is created or destroyed; atoms are just rearranged as the atoms change partners to form new compounds. – If there are 3 atoms of oxygen in the reactants, there MUST be 3 atoms of oxygen in the products. – Number of each atom in reactants = number of each atom in products. • The law of conservation of mass: – Mass of reactants = mass of products If you could collect and measure all of the exhaust from this car, you would find that mass of reactants (gas + O2) = mass of products (exhaust). (c) McGraw Hill Ryerson 2007 • The simplest form of chemical equation is a word equation. – Potassium metal + oxygen gas potassium oxide • A skeleton equation shows the formulas of the elements/compounds. – A skeleton equation shows which atoms are involved, but not how many molecules are involved. • K + O2 K2O • A balanced chemical equation shows all atoms and the coefficients tells us how many molecules (and atoms) there are. – Balancing ensures that the number of each atom is the same on both sides of the reaction arrow. 4K K K K K + O2 O O 2K2O K O K K O K • Using the law of conservation of mass, we can count atoms to balance the number of atoms in chemical equations. – Word equation: methane + oxygen water + carbon dioxide – Skeleton equation: CH4 + O2 H2O + CO2 • To balance the compounds, take note of how many atoms of each element occur on each side of the reaction arrow. (c) McGraw Hill Ryerson 2007 See Page 207 Skeleton equation: The same number of atoms must be on each side. Balanced equation: (c) McGraw Hill Ryerson 2007 CH4 + O2 H2O + CO2 Carbon = 1 Hydrogen = 4 Oxygen = 2 Carbon = 1 Hydrogen = 2 Oxygen = 3 CH4 + 2O2 2H2O + CO2 Carbon = 1 Carbon = 1 Hydrogen = 4 Oxygen = 4 Hydrogen = 4 Oxygen = 4 See Page 207 Balancing Equations hydrogen H2 + oxygen + O2 water H2O H Reactants 2 Products 2 O 2 1 Balancing Equations hydrogen + oxygen H2 + O2 hydrogen peroxide H2O2 YOU CANNOT CHANGE THE SUBSCRIPTS H O Reactants 2 2 Products 2 2 Balancing Equations hydrogen + oxygen H2 + H O O2 Reactants 2 2 water 2 H2O Products 2 1 Balancing Equations hydrogen + oxygen H2 + H O O2 Reactants 2 2 water 2 H2O Products 4 2 Balancing Equations hydrogen + oxygen 2 H2(g) + H O water O2 (g) 2 H2O (l) Reactants 4 2 Products 4 2 • Balance chemical equations by following these steps: – Trial and error will work but can be very inefficient. • USE A TABLE (write atoms underneath reactants and products) • If they look the same on both sides of the equation, treat polyatomic ions (such as SO42–) as a group & balance them as such. • If ‘OH’ and H2O are in the equation, write water as HOH. • Balance one compound at a time & rewrite the # of atoms in the chart as things change. • Only add coefficients; NEVER change subscripts!!! • If H and O appear in more than one place, attempt to balance them LAST. – Balance everything that isn’t ‘H’ or ‘O’ 1st. – Balance the ‘H’s 2nd to last. – Balance the ‘O’s last. – Always double-check after you think you are finished. – CHECK YOUR ANSWERS!!! See pages 209 - 211 (c) McGraw Hill Ryerson 2007 http://www.authorstream.com/Presentation/kellymdeters-86103-section2-7-balancing-chemical-equations-education-ppt-powerpoint/ • Balance the following: – Fe + Br2 FeBr3 – Sn(NO2)4 + K3PO4 KNO2 + Sn3 (PO4)4 – C2H6 + O2 CO2 + H2O ____Ba + ____H2O ____CO2 + ____H2O ____Fe2O3 + ____C ____Fe + ____H2O ____Ba(OH)2 + ____H2 ____H2CO3 ____Fe + ____CO ____H2 + ____Fe2O3 • If you don’t transform your word into a skeleton equation properly, you won’t be able to balance the equation correctly. – Change chemical names into chemical formulas. 4 types: • Simple ionic compounds • Multivalent ionic compounds • Ionic compounds with polyatomic ions • Covalent compound • Be careful of diatomic elements -remember the special seven!! H2, N2, O2, F2, Cl2, Br2, I2 See page 208 (c) McGraw Hill Ryerson 2007 Several common covalent molecules containing hydrogen have common names that you should know and MEMORIZE!! methane = CH4 glucose = C6H12O6 ethane = C2H6 ammonia = NH3 See page 208 (c) McGraw Hill Ryerson 2007 Example #1: Word Equation: Solutions of lead nitrate react with potassium iodide to produce solid lead iodide and a solution of potassium nitrate. Skeleton Equation: Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) Balanced Equation: Pb(NO3)2(aq) + 2KI(aq) PbI2(s) + 2KNO3(aq) Example #2: Word Equation: Copper reacts with hydrogen nitrate to produce copper (II) nitrate plus hydrogen. Skeleton Equation: Cu + H(NO3) Balanced Equation: Cu + 2H(NO3) Cu(NO3)2 + H2 Cu(NO3)2 + H2 See page 208 (c) McGraw Hill Ryerson 2007