Unit 2: Chemical Reactions 4.1: Intro to Chemical Reactions
advertisement
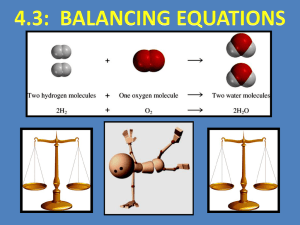
Unit 2: Chemical Reactions 4.1: Intro to Chemical Reactions Chemical Reaction Process where one or more substances change into one or more new substances Evidence of a Chemical Reaction 1. There is an unexpected colour change 2. Energy is released or absorbed, often in the form of heat or light 3. A gas (bubbles) is produced 4. A precipitate forms (two liquids mixed together form a solid and a liquid) Counting Atoms and Molecules # of atoms: subscript ◦ H2O = 2 hydrogen atoms, 1 oxygen atom # of molecules: coefficient ◦ 2 H2O = 2 molecules, 4 hydrogen atoms, 2 oxygen atoms Try: 3 Al2(CO3)3 ◦ # molecules = 3 ◦ # atoms: 6 aluminum, 9 carbon, 27 oxygen Symbols and Terms Used In Chemical Reactions 1. Word Equations: use words ◦ Example: Iron + sulfur Reactants (starting substances) iron(II) sulfide Yields/ produces Products (ending substances) 2. Chemical Equations: use chemical formulas May be unbalanced (skeleton) or balanced Example: Fe (s) + S (s) State FeS (s) Symbols and Terms Used In Chemical Reactions State symbols: (s) = Solid (l) = Liquid (g) = Gas (aq) = Aqueous (dissolved in water) Balancing Chemical Equations Follow the Law of Conservation of Mass: ◦ Mass reactants = mass products Add coefficients in front of the formulas to balance the equation! ** only whole numbers can be used ** you CANNOT change subscripts ** you must REDUCE the coefficients Balancing Chemical Equations TIPS! Balance any element that occurs more than once on one side of the equation last (often H and O) Keep polyatomic ions together as a group if it remains unchanged on both sides of the reaction ALWAYS do one last check to make sure everything is balanced! Practice: Balance the following skeleton equations: 1. N2 + 2. Fe + H2 NH3 O2 Fe2O3 3. Be + Al2O3 BeO + 4. Mg + HNO3 H2 + 5. CH4 + O2 CO2 + Al Mg(NO3)2 H2O