Gas PPT
advertisement

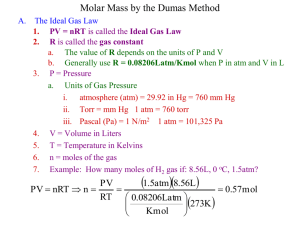
GASES Chapter 12 The Atmosphere The atmosphere is a gaseous solution of nitrogen, N2, and oxygen, O2. The atmosphere both supports life and acts as a waste receptacle for the exhaust gases that accompany many industrial processes leading to many types of pollution, including smog and acid rain. A Gas - Uniformly fills any container. - Mixes completely with any other gas - Exerts pressure on its surroundings. - Is easily compressed. 05_47 Vacuum h = 760mm Hg for standard atmosphere Simple barometer invented by Evangelista Torricelli. A barometer is used to measure atmospheric pressure. Atmospheric Pressure Atmospheric pressure results from the mass of the air being pulled toward the center of the earth by gravity--the weight of the air. At sea level, atmospheric pressure is 760mm. Atmospheric pressure varies with altitude-at Breckenridge, CO (elevation 9600 ft), the pressure is 520 mm. Figure 12.1: The pressure exerted by the gases in the atmosphere can demonstrated by boiling water in a can Pressure - is equal to force/unit area - SI units = Newton/meter2 = 1 Pascal (Pa) - 1 standard atmosphere = 101,325 Pa - 1 standard atmosphere = 1 atm = 760 mm Hg = 760 torr Atmospheric pressure (Patm) 05_48 Atmospheric pressure (Patm) h Gas pressure (Pgas) less than atmospheric pressure (Pgas) = (Patm) - h (a) h Gas pressure (Pgas) greater than atmospheric pressure (Pgas) = (Patm) + h (b) A simple manometer is a device for measuring the pressure of a gas in a container. Pressure Unit Conversions The pressure of a tire is measured to be 28 psi. What would the pressure be in atmospheres, torr, and pascals. (28 psi)(1.000 atm/14.69 psi) = 1.9 atm (28 psi)(1.000 atm/14.69 psi)(760.0 torr/1.000atm) = 1.4 x 103 torr (28 psi)(1.000 atm/14.69 psi)(101,325 Pa/1.000 atm) = 1.9 x 105 Pa 05_1541 Pext Pext Volume is decreased Volume of a gas decreases as pressure increases at constant temperature 05_50 40 slope = k V(in3) P (in Hg) 100 50 20 P P 2 0 0 20 40 60 0 0.01 0.02 0.03 1/P (in Hg) V 2V V(in3) (a) P vs V (b) V vs 1/P BOYLE’S LAW DATA Figure 12.6: Illustration of Boyle’s law Boyle’s * Law Robert Boyle -- Irish scientist (1627-1691) (Pressure)( Volume) = Constant (T = constant) P1V1 = P2V2 V 1/P (T = constant) (T = constant) (*Holds precisely only at very low pressures.) The temperature and amount of gas must remain unchanged. A gas that strictly obeys Boyle’s Law is called an ideal gas. Boyle’s Law Calculations A 1.5-L sample of gaseous CCl2F2 has a pressure of 56 torr. If the pressure is changed to 150 torr, will the volume of the gas increase or decrease? What will the new volume be? Decrease P1 = 56 torr P2 = 150 torr V1 = 1.5 L V2 = ? V1P1 = V2P2 V2 = V1P1/P2 V2 = (1.5 L)(56 torr)/(150 torr) V2 = 0.56 L Boyle’s Law Calculations In an automobile engine the initial cylinder volume is 0.725 L. After the piston moves up, the volume is 0.075 L. The mixture is 1.00 atm, what is the final pressure? P1 = 1.00 atm P2 = ? V1 = 0.725 L V1P1 = V2P2 P2 = V1P1/V2 P2 = (0.725 L)(1.00 atm)/(0.075 L) P2 = 9.7 atm V2 = 0.075 L Is this answer reasonable? He 05_53 6 5 CH4 V(L) 4 3 H2O 2 H2 1 N2O -300 -200 -273.2 ºC -100 0 100 200 300 T(ºC) Plot of V vs. T(oC) for several gases 05_1543 Pext Pext Energy (heat) added Volume of a gas increases as heat is added when pressure is held constant. Charles’s Law Jacques Charles -- French physicist (17461823) The volume of a gas is directly proportional to temperature, and extrapolates to zero at zero Kelvin. V = bT (P = constant) y = mx + b b = a proportionality constant Charles’s Law V1 V2 T1 T2 ( P constant) Temperature must be in Kelvin!! Charles’s Law Calculations A 2.0-L sample of air is collected at 298 K and then cooled to 278 K. The pressure is held constant. Will the volume increase or decrease? What will the new volume be? Decrease V1 = 2.0 L V2 = ? T1 = 298 K T2 = 278 K V1/V2 = T1/T2 V2 = V1 T2/T1 V2 = (2.0 L)(278 K)/(298 K) V2 = 1.9 L Charles’s Law Calculations Consider a gas with a a volume of 0.675 L at 35 oC and 1 atm pressure. What is the temperature (in Co) of the gas when its volume is 0.535 L at 1 atm pressure? V1/V2 = T1/T2 V1 = 0.675 L T2 = T1 V2/V1 T2 = (308 K)(0.535 L)/(0.675 L) V2 = 0.535 L T2 = 244 K -273 T1 = 35 oC + 273 = 308 K T2 = - 29 oC T2 = ? 05_1544 Pext Pext Gas cylinder Moles of gas increases Pext Increase volume to return to original pressure At constant temperature and pressure, increasing the moles of a gas increases its volume. Figure 12.11: The total pressure of a mixture of gases depends on the number of moles of gas particles (atoms or molecules) present Avogadro’s Law For a gas at constant temperature and pressure, the volume is directly proportional to the number of moles of gas (at low pressures). V = an y = mx + b a = proportionality constant V = volume of the gas n = number of moles of gas AVOGADRO’S LAW V1 n1 V2 n 2 Temperature and pressure must remain constant!! AVOGADRO’S LAW A 12.2 L sample containing 0.50 mol of oxygen gas, O2, at a pressure of 1.00 atm and a temperature of 25 oC is converted to ozone, O3, at the same temperature and pressure, what will be the volume of the ozone? 3 O2(g) ---> 2 O3(g) (0.50 mol O2)(2 mol O3/3 mol O2) = 0.33 mol O3 V1 = 12.2 L V2 = ? n1 = 0.50 mol n2 = 0.33 mol V1/V2 = n1/n2 V2 = V1 n2/n1 V2 = (12.2 L)(0.33 mol)/(0.50 mol) V2 = 8.1 L COMBINED GAS LAW What will be the new volume of a gas under the following conditions? V1 = 3.48 L V2 = ? P1 = 0.454 atm P2 = 0.616 atm T1 = - 15 oC + 273 = 258 K T2 = 36 oC + 273 T2= 309 K V1/ V2 = P2 T1/ P1 T2 V2 = V1P1T2/P2T1 V2 = (309 K)(0.454 atm)(3.48 L) (258 K)(0.616 atm) V2 = 3.07 L 05_1542 Pext Pext Temperature is increased Pressure exerted by a gas increases as temperature increases provided volume remains constant. If the volume of a gas is held constant, then V1 / V2 = 1. Therefore: P1 / P2 = T1 / T2 Pressure and Kelvin temperature are directly proportional. IDEAL GAS 1. Molecules are infinitely far apart. 2. Zero attractive forces exist between the molecules. 3. Molecules are infinitely small--zero molecular volume. What is an example of an ideal gas? REAL GAS 1. Molecules are relatively far apart compared to their size. 2. Very small attractive forces exist between molecules. 3. The volume of the molecule is small compared to the distance between molecules. What is an example of a real gas? Real vs. Ideal Gases When p 1 atm and T O oC, real gases approximate ideal gases. Ideal Gas Law - An equation of state for a gas. - “state” is the condition of the gas at a given time. PV = nRT Ideal Gas Law PV = nRT R = proportionality constant = 0.08206 L atm mol P = pressure in atm V = volume in liters n = moles T = temperature in Kelvins Holds closely at P 1 atm Ideal Gas Law Calculations A sample of hydrogen gas, H2, has a volume of 8.56 L at a temperature of O oC and a pressure of 1.5 atm. Calculate the number of moles of hydrogen present. p = 1.5 atm pV = nRT V = 8.56 L n = pV/RT R = 0.08206 Latm/molK n = (1.5 atm)(8.56L) (0.08206 Latm/molK)(273K) n=? n = 0.57 mol T = O oC + 273 T = 273K Ideal Gas Law Calculations A 1.5 mol sample of radon gas has a volume of 21.0 L at 33 oC. What is the pressure of the gas? p=? pV = nRT p = nRT/V V = 21.0 L p = (1.5mol)(0.08206Latm/molK)(306K) n = 1.5 mol (21.0L) T = 33 oC + 273 p = 1.8 atm T = 306 K R = 0.08206 Latm/molK Dalton’s Law of Partial Pressures For a mixture of gases in a container, the total pressure exerted is the sum of the partial pressures of the gases present. PTotal = P1 + P2 + P3 + . . . Dalton’s Law of Partial Pressures Calculations A mixture of nitrogen gas at a pressure of 1.25 atm, oxygen at 2.55 atm, and carbon dioxide at .33 atm would have what total pressure? PTotal = P1 + P2 + P3 PTotal = 1.25 atm + 2.55 atm + .33 atm Ptotal = 4.13 atm Water Vapor Pressure 2KClO3(s) ----> 2KCl(s) + 3O2(g) When a sample of potassium chlorate is decomposed and the oxygen produced collected by water displacement, the oxygen has a volume of 0.650 L at a temperature of 22 oC. The combined pressure of the oxygen and water vapor is 754 torr (water vapor pressure at 22 oC is 21 torr). How many moles of oxygen are produced? Pox = Ptotal - PHOH Pox = 754 torr - 21 torr pox = 733 torr Water Vapor Pressure Continued p = (733 torr)(1 atm/760 torr) p = 0.964 atm V = 0.650 L n=? T = 22 oC + 273 pV = nRT n = pV/RT n = (0.964 atm)(0.650 L) (0.08206 Latm/molK)(295K) n = 2.59 x 10-2 mol T = 295 K R = 0.08206 Latm/molK Kinetic Molecular Theory 1. Volume of individual particles is zero. 2. Collisions of particles with container walls cause pressure exerted by gas. 3. Particles exert no forces on each other. 4. Average kinetic energy Kelvin temperature of a gas. 5. Gases consist of tiny particles (atoms or molecules. Relative number of O2 molecules with given velocity 05_58 0 4 x 102 8 x102 Molecular velocity (m/s) Plot of relative number of oxygen molecules with a given velocity at STP (Boltzmann Distribution). Relative number of N2 molecules with given velocity 05_59 273 K 1273 K 2273 K 0 1000 2000 Velocity (m/s) 3000 Plot of relative number of nitrogen molecules with a given velocity at three different temperatures. Kinetic Molecular Theory As the temperature of a gas increases, the velocity of the molecules increases. Molecules at a higher temperature have greater KE and hit the walls of the container harder and more often exerting more pressure. If the container is not rigid, the container and volume of the gas will expand. Standard Temperature and Pressure “STP” P = 1 atmosphere T = C The molar volume of an ideal gas is 22.42 liters at STP Molar Volume pV = nRT V = nRT/p V = (1.00 mol)(0.08206 Latm/molK)(273K) (1.00 atm) V = 22.4 L Figure 12.12: The production of oxygen by thermal decomposition of KClO3 GAS STOICHIOMETRY Not at STP Calculate the volume of oxygen gas produced at 1.00 atm and 25 oC by the complete decomposition of 10.5 g of potassium chlorate. 2KClO3(s) ----> 2KCl(s) + 3O2(g) (10.5g KClO3)(1mol/122.6 g)(3 mol O2/2mol KClO3) = 1.28 x 10-1 mol O2 Gas Stoichiometry Not at STP (Continued) p = 1.00 atm V=? n = 1.28 x 10-1 mol R = 0.08206 Latm/molK T = 25 oC + 273 = 298 K pV = nRT V = nRT/p V = (1.28 x 10-1mol)(0.08206Latm/molK)(298K) (1.00 atm) V = 3.13 L O2 Gases at STP A sample of nitrogen gas has a volume of 1.75 L at STP. How many moles of N2 are present? (1.75L N2)(1.000 mol/22.4 L) = 7.81 x 10-2 mol N2 Gas Stoichiometry at STP Quicklime, CaO, is produced by heating calcium carbonate, CaCO3. Calculate the volume of CO2 produced at STP from the decomposition of 152 g of CaCO3. CaCO3(s) ---> CaO(s) + CO2(g) (152g CaCO3)(1 mol/100.1g)(1mol CO2/1mol CaCO3) (22.4L/1mol) = 34.1L CO2 Note: This method only works when the gas is at STP!!!!! Chemistry is a gas!!