Poster.ppt
advertisement
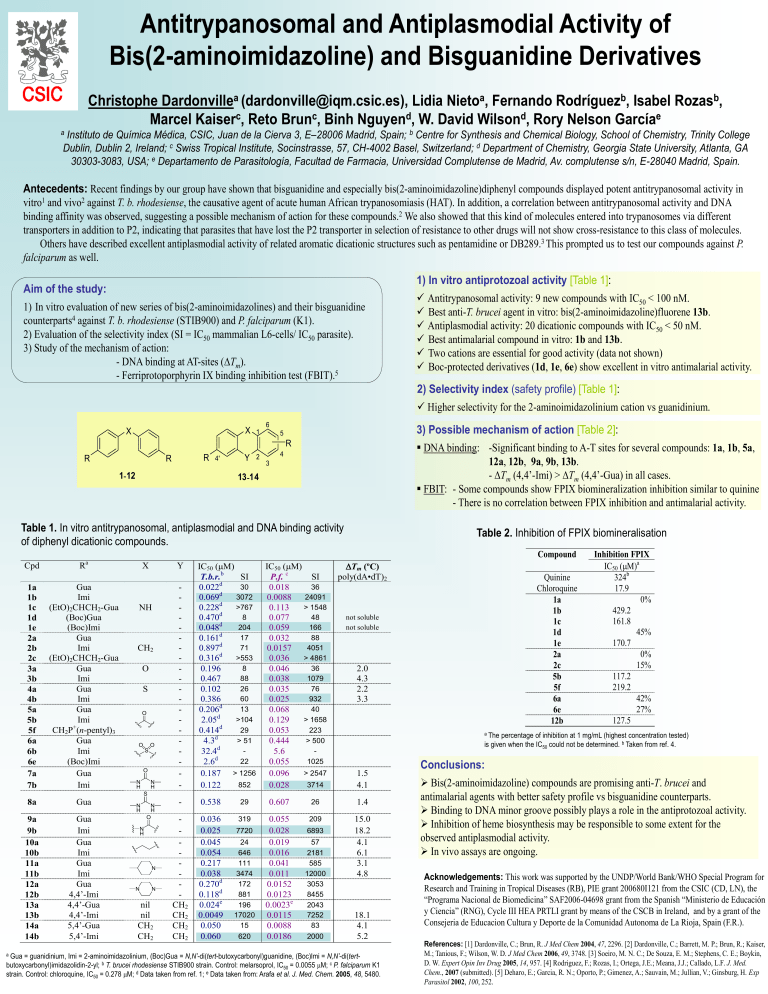
Antitrypanosomal and Antiplasmodial Activity of Bis(2-aminoimidazoline) and Bisguanidine Derivatives Christophe Dardonvillea (dardonville@iqm.csic.es), Lidia Nietoa, Fernando Rodríguezb, Isabel Rozasb, Marcel Kaiserc, Reto Brunc, Binh Nguyend, W. David Wilsond, Rory Nelson Garcíae Instituto de Química Médica, CSIC, Juan de la Cierva 3, E–28006 Madrid, Spain; b Centre for Synthesis and Chemical Biology, School of Chemistry, Trinity College Dublin, Dublin 2, Ireland; c Swiss Tropical Institute, Socinstrasse, 57, CH-4002 Basel, Switzerland; d Department of Chemistry, Georgia State University, Atlanta, GA 30303-3083, USA; e Departamento de Parasitología, Facultad de Farmacia, Universidad Complutense de Madrid, Av. complutense s/n, E-28040 Madrid, Spain. a Antecedents: Recent findings by our group have shown that bisguanidine and especially bis(2-aminoimidazoline)diphenyl compounds displayed potent antitrypanosomal activity in vitro1 and vivo2 against T. b. rhodesiense, the causative agent of acute human African trypanosomiasis (HAT). In addition, a correlation between antitrypanosomal activity and DNA binding affinity was observed, suggesting a possible mechanism of action for these compounds.2 We also showed that this kind of molecules entered into trypanosomes via different transporters in addition to P2, indicating that parasites that have lost the P2 transporter in selection of resistance to other drugs will not show cross-resistance to this class of molecules. Others have described excellent antiplasmodial activity of related aromatic dicationic structures such as pentamidine or DB289.3 This prompted us to test our compounds against P. falciparum as well. 1) In vitro antiprotozoal activity [Table 1]: Aim of the study: 1) In vitro evaluation of new series of bis(2-aminoimidazolines) and their bisguanidine counterparts4 against T. b. rhodesiense (STIB900) and P. falciparum (K1). 2) Evaluation of the selectivity index (SI = IC50 mammalian L6-cells/ IC50 parasite). 3) Study of the mechanism of action: - DNA binding at AT-sites (Tm). - Ferriprotoporphyrin IX binding inhibition test (FBIT).5 Antitrypanosomal activity: 9 new compounds with IC50 < 100 nM. Best anti-T. brucei agent in vitro: bis(2-aminoimidazoline)fluorene 13b. Antiplasmodial activity: 20 dicationic compounds with IC50 < 50 nM. Best antimalarial compound in vitro: 1b and 13b. Two cations are essential for good activity (data not shown) Boc-protected derivatives (1d, 1e, 6e) show excellent in vitro antimalarial activity. 2) Selectivity index (safety profile) [Table 1]: Higher selectivity for the 2-aminoimidazolinium cation vs guanidinium. X X 6 1 3) Possible mechanism of action [Table 2]: 5 R R R R 4' 1- 12 Y 2 DNA binding: -Significant binding to A-T sites for several compounds: 1a, 1b, 5a, 12a, 12b, 9a, 9b, 13b. - Tm (4,4’-Imi) > Tm (4,4’-Gua) in all cases. FBIT: - Some compounds show FPIX biomineralization inhibition similar to quinine - There is no correlation between FPIX inhibition and antimalarial activity. 4 3 13 -14 Table 1. In vitro antitrypanosomal, antiplasmodial and DNA binding activity of diphenyl dicationic compounds. Table 2. Inhibition of FPIX biomineralisation Compound Cpd 1a 1b 1c 1d 1e 2a 2b 2c 3a 3b 4a 4b 5a 5b 5f 6a 6b 6e 7a 7b Ra Gua Imi (EtO)2CHCH2-Gua (Boc)Gua (Boc)Imi Gua Imi (EtO)2CHCH2-Gua Gua Imi Gua Imi Gua Imi CH2P+(n-pentyl)3 Gua Imi (Boc)Imi Gua Imi X Y NH CH2 O S O O O S O N H N H - IC50 (mM) T.b.r.b SI 30 0.022d 3072 0.069d >767 0.228d 8 0.470d 204 0.048d 17 0.161d 71 0.897d >553 0.316d 8 0.196 88 0.467 26 0.102 60 0.386 13 0.206d >104 2.05d 29 0.414d > 51 4.3d 32.4d 22 2.6d > 1256 0.187 852 0.122 IC50 (mM) P.f. c SI 36 0.018 24091 0.0088 > 1548 0.113 48 0.077 166 0.059 88 0.032 4051 0.0157 > 4861 0.036 36 0.046 1079 0.038 76 0.035 932 0.025 40 0.068 > 1658 0.129 223 0.053 > 500 0.444 5.6 1025 0.055 > 2547 0.096 3714 0.028 Tm (ºC) poly(dA•dT)2 not soluble not soluble 2.0 4.3 2.2 3.3 a The percentage of inhibition at 1 mg/mL (highest concentration tested) is given when the IC50 could not be determined. b Taken from ref. 4. 1.5 4.1 S 8a Gua 9a 9b 10a 10b 11a 11b 12a 12b 13a 13b 14a 14b Gua Imi Gua Imi Gua Imi Gua 4,4’-Imi 4,4’-Gua 4,4’-Imi 5,4’-Gua 5,4’-Imi N H N H O N H N N N nil nil CH2 CH2 - 0.538 29 0.607 26 1.4 CH2 CH2 CH2 CH2 0.036 0.025 0.045 0.054 0.217 0.038 0.270d 0.118d 0.024e 0.0049 0.050 0.060 319 0.055 0.028 0.019 0.016 0.041 0.011 0.0152 0.0123 0.0023e 0.0115 0.0088 0.0186 209 15.0 18.2 4.1 6.1 3.1 4.8 7720 24 646 111 3474 172 881 196 17020 15 620 6893 57 2181 585 12000 3053 8455 2043 7252 83 2000 18.1 4.1 5.2 Gua = guanidinium, Imi = 2-aminoimidazolinium, (Boc)Gua = N,N’-di(tert-butoxycarbonyl)guanidine, (Boc)Imi = N,N’-di(tertbutoxycarbonyl)imidazolidin-2-yl; b T. brucei rhodesiense STIB900 strain. Control: melarsoprol, IC50 = 0.0055 mM; c P. falciparum K1 strain. Control: chloroquine, IC50 = 0.278 mM; d Data taken from ref. 1; e Data taken from: Arafa et al. J. Med. Chem. 2005, 48, 5480. a Quinine Chloroquine 1a 1b 1c 1d 1e 2a 2c 5b 5f 6a 6e 12b Inhibition FPIX IC50 (mM)a 324b 17.9 0% 429.2 161.8 45% 170.7 0% 15% 117.2 219.2 42% 27% 127.5 Conclusions: Bis(2-aminoimidazoline) compounds are promising anti-T. brucei and antimalarial agents with better safety profile vs bisguanidine counterparts. Binding to DNA minor groove possibly plays a role in the antiprotozoal activity. Inhibition of heme biosynthesis may be responsible to some extent for the observed antiplasmodial activity. In vivo assays are ongoing. Acknowledgements: This work was supported by the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (RB), PIE grant 200680I121 from the CSIC (CD, LN), the “Programa Nacional de Biomedicina” SAF2006-04698 grant from the Spanish “Ministerio de Educación y Ciencia” (RNG), Cycle III HEA PRTLI grant by means of the CSCB in Ireland, and by a grant of the Consejeria de Educacion Cultura y Deporte de la Comunidad Autonoma de La Rioja, Spain (F.R.). References: [1] Dardonville, C.; Brun, R. J Med Chem 2004, 47, 2296. [2] Dardonville, C.; Barrett, M. P.; Brun, R.; Kaiser, M.; Tanious, F.; Wilson, W. D. J Med Chem 2006, 49, 3748. [3] Soeiro, M. N. C.; De Souza, E. M.; Stephens, C. E.; Boykin, D. W. Expert Opin Inv Drug 2005, 14, 957. [4] Rodriguez, F.; Rozas, I.; Ortega, J.E.; Meana, J.J.; Callado, L.F. J. Med. Chem., 2007 (submitted). [5] Deharo, E.; Garcia, R. N.; Oporto, P.; Gimenez, A.; Sauvain, M.; Jullian, V.; Ginsburg, H. Exp Parasitol 2002, 100, 252.