C344 Homework #4 Kinetics
advertisement

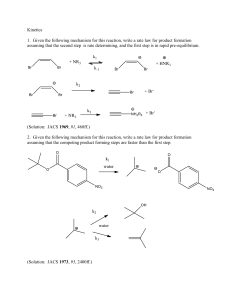
C344 Homework #4 Kinetics 1. Given the following mechanism for this reaction, write a rate law for product formation assuming that the second step is rate determining, and the first step is in rapid pre-equilibrium. (Solution: JACS 1969, 91, 468ff.) 2. Given the following mechanism for this reaction, write a rate law for product formation assuming that the competing product forming steps are faster than the first step. (Solution: JACS 1973, 95, 2400ff.) 3. Given the following mechanism for this reaction, write a rate law for product formation with no assumptions concerning the relative rates of the steps. (Solution: JACS 1966, 88, 4749ff.) 4. Reactions of dialkylaluminum hydrides with alkynes give addition products: R2Al R Al H + R C C H R' R R R' The rate expression was determined to be: − 𝑑[𝐴] 𝑑𝑡 = k[A][(R2AlH)3]1/3 Propose a mechanism that could account for the overall four-thirds order kinetics and the appearance of the dialkyl hydride concentration to the 1/3 power. (Solution: Eisch and Rhee, JACS 1974, 96, 7276ff.) 5. A series of 18O-labelled sulfonate esters was prepared, and the extent of 18O scrambling which accompanies substitution was measured. The rate of 18O exchange was compared with the rate of solvolysis. Disucss the variation of the ratio of the rate constant of substituion compared to the rate constant of exchange (ksub/kex). O O O O R O S O 18 18 R CF3 R Ph O18 S Ph + O CF 3 O O18 O HO R CH3 CF3 substitution product exchange product ksub 3.6 x 10-5 kex 7.9 x 10-6 ksub/kex 4.6 3.8 x 10-3 8.5 x 10-4 4.5 1.5 x 10-3 1.8 x 10-3 0.83 CH CH3 (Solution: Parsadisi and Bunnett, JACS 1981, 103, 946ff.)