kineticshomework

Kinetics
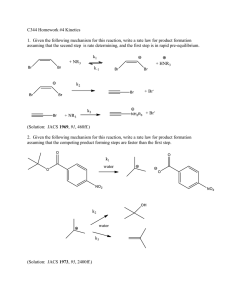
1. Given the following mechanism for this reaction, write a rate law for product formation assuming that the second step is rate determining, and the first step is in rapid pre-equilibrium.
(Solution: JACS 1969 , 91 , 468ff.)
2. Given the following mechanism for this reaction, write a rate law for product formation assuming that the competing product forming steps are faster than the first step.
(Solution: JACS 1973 , 95 , 2400ff.)
3. Given the following mechanism for this reaction, write a rate law for product formation with no assumptions concerning the relative rates of the steps.
(Solution: JACS 1966 , 88 , 4749ff.)
4. Reactions of dialkylaluminum hydrides with alkynes give addition products:
R
2
Al
R
H
Al H +
R C C R'
R
R R'
The rate expression was determined to be:
− 𝑑[𝐴] 𝑑𝑡
= k[A][(R
2
AlH)
3
] 1/3
Propose a mechanism that could account for the overall four-thirds order kinetics and the appearance of the dialkyl hydride concentration to the 1/3 power. (Solution: Eisch and Rhee,
JACS 1974 , 96 , 7276ff.)
5. A series of
18
O-labelled sulfonate esters was prepared, and the extent of
18
O scrambling which accompanies substitution was measured. The rate of
18
O exchange was compared with the rate of solvolysis. Disucss the variation of the ratio of the rate constant of substituion compared to the rate constant of exchange (k sub
/k ex
).
O
O
O
18 O
18
O CF
3
R
R O S Ph
R O
18
S Ph +
O CF
3
O
O
18
HO CF
3
O exchange product substitution product
R
CH
3 k sub
3.6 x 10
-5 k ex
7.9 x 10
-6 k sub
/k ex
4.6
CH
CH
3
3.8 x 10 -3 8.5 x 10 -4 4.5
1.5 x 10
-3
1.8 x 10
-3
0.83
(Solution: Parsadisi and Bunnett, JACS 1981 , 103 , 946ff.)